What Is HTA? | HTA News & Events | Resource Library | ISPOR Global HTA Governance | Policy Interchange | Training and Development
The Health Technology Assessment Process
In addition to the first step of defining the HTA process, the steps to conducting a thorough health technology assessment include:
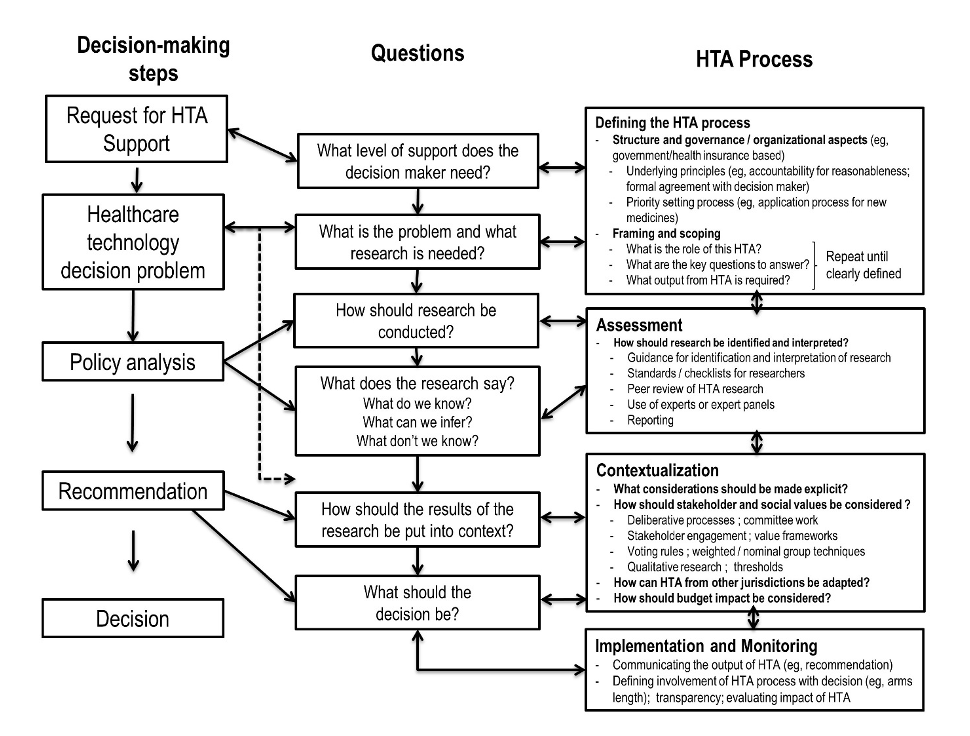
The following featured HTA resources are organized by the key steps for conducting an HTA. This icon  indicates ISPOR created content.
indicates ISPOR created content.
DEFINING THE HTA PROCESS
Defining the HTA Process: Structure/Governance/Organizational Aspects of HTA
- A Framework for Good Governance in the Pharmaceutical Sector. The Hashemite Kingdom of Jordan
- Governance for sustainable human development: a UNDP policy document. New York: United Nations Development Programme; 1997.
- Governance indicators: where are we, where should we be going? The World Bank; 2007 Oct p. 1. Report No.: WPS4370.
- Handbook on HTA Capacity Building (EUnetHTA)
- HTA country survey of National Authorities (World Health Organization [WHO]).
- Policy for the HTA Core Model® and core HTA information (EUnetHTA)
- Strengthening health system governance: better policies, stronger performance. Maidenhead, Berkshire, England: Open University Press; 2016.
- The world health report 2000 – Health systems: improving performance (WHO)
Defining the HTA Process: Framework/Principles for the HTA Process
- Accountability for reasonableness.
- A Comparison of Reimbursement Recommendations by European HTA Agencies: Is There Opportunity for Further Alignment?
- Assessing the Added Value of Health Technologies: Reconciling Different Perspectives.
- Can we reliably benchmark health technology assessment organizations?
- Enhancing Fairness and Legitimacy.
- Guiding principles for good practices in hospital-based health technology assessment units.
- Health technology assessment agencies: an international overview of organizational aspects.
- Health technology assessment, deliberative process, and ethically contested issues.
- Health technology assessment for resource allocation decisions: are key principles relevant for Latin America?
- Health technology assessment toolkit (iDSI)
- Key principles for the improved conduct of health technology assessments for resource allocation decisions.
- Mapping of health technology assessment in selected countries.
- The impact of culture, values, and institutional context on the methods and use of economic evaluation.
- The role of health technology assessment on pharmaceutical reimbursement in selected middle-income countries.
- Towards Integrated Health Technology Assessment for Improving Decision Making in Selected Countries.
- Toward international good practices in health technology assessment.
Defining the HTA Process: Priority Setting Process
- AHRQ series paper 3: identifying, selecting, and refining topics for comparative effectiveness systematic reviews: AHRQ and the effective health-care program.
- Choosing health technology assessment and systematic review topics: the development of priority-setting criteria for patients’ and consumers’ interests.
- Emerging health technologies: informing and supporting health policy early.
- How to choose health technologies to be assessed by HTA? A review of criteria for priority setting.
- Priority setting for health technology assessment. Theoretical considerations and practical approaches. Priority setting Subgroup of the EUR-ASSESS Project.
- Priority setting for health technology assessment at CADTH.
- Priority setting for health technology assessments: a systematic review of current practical approaches.
- The health systems’ priority setting criteria for selecting health technologies: A systematic review of the current evidence.
- The Refinement of Topics for Systematic Reviews: Lessons and Recommendations From the Effective Health Care Program. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
Defining the HTA Process: Framing and Scoping
- An integrated perspective on the assessment of technologies: Integrate-HTA.
- Formulating questions and locating primary studies for inclusion in systematic reviews.
- Guidance for conducting systematic scoping reviews.
- Health technology assessment handbook. Copenhagen: Danish Centre for Health Technology Assessment; 2001
- HTA Core Model® (EUnetHTA)
- Lay and professional stakeholder involvement in scoping palliative care issues: Methods used in seven European countries.
- Technology appraisal guidance (NICE)
- Using scoping literature reviews as a means of understanding and interpreting existing literature.
ASSESSMENT
Assessment: Identifying and Interpreting Individual Studies
- A Checklist for Medication Compliance and Persistence Studies Using Retrospective Databases

- A Checklist for Retrospective Database Studies

- Analytic Methods to Improve Causal Inference From Non-Randomized Studies of Treatment Effects Using Secondary Databases

- An integrated perspective on the assessment of technologies: INTEGRATE-HTA.
- Approaches to Mitigate Bias and Confounding in the Design of Non-Randomized Studies of Treatment Effects Using Secondary Databases

- A Questionnaire to Assess the Relevance and Credibility of Observational Studies to Inform Healthcare Decision Making

- Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
- Cochrane Qualitative and Implementation Methods Group guidance series—paper 1: introduction.
- Cochrane Qualitative and Implementation Methods Group guidance series-paper 2: methods for question formulation, searching, and protocol development for qualitative evidence synthesis
- Cochrane Qualitative and Implementation Methods Group guidance paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings.
- Cochrane Qualitative and Implementation Methods Group guidance paper 4: methods for assessing evidence on intervention implementation.
- Cochrane Qualitative and Implementation Methods Group guidance paper 5: methods for integrating qualitative and implementation evidence within intervention effectiveness reviews.
- Cochrane Qualitative and Implementation Methods Group guidance paper 6: reporting guidelines for qualitative, implementation, and process evaluation evidence syntheses.
- Complex health care interventions: characteristics relevant for ethical analysis in health technology assessment.
- Complexity of the paradigms present in quality criteria of qualitative research grids.
- Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute.
- Conjoint Analysis Applications in Health—A Checklist

- Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups.
- Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Resources

- Constructing Experimental Designs for Discrete-Choice Experiments

- Content Validity—Establishing and Reporting the Evidence in Newly Developed Patient-Reported Outcomes Instruments for Medical Product Evaluation—Assessing Respondent Understanding

- Content Validity—Establishing and Reporting the Evidence in Newly Developed Patient-Reported Outcomes (PRO) Instruments for Medical Product Evaluation—Eliciting Concepts for a New PRO Instrument

- Cost-Effectiveness Analysis Alongside Clinical Trials II

- Cost-Effectiveness in Health and Medicine. 02 edition. Oxford; New York: Oxford University Press; 2016.
- Coverage with Evidence Development, Only in Research, Risk Sharing, or Patient Access Scheme? A Framework for Coverage Decisions.
- Coverage with evidence development: the Ontario experience.
- Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria.
- Current state of ethics literature synthesis: a systematic review of reviews.
- Defining, Reporting, and Interpreting Non-Randomized Studies of Treatment Effects Using Secondary Databases

- Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
- Different methods for ethical analysis in health technology assessment: an empirical study.
- Economic modelling of diagnostic and treatment pathways in National Institute for Health and Care Excellence clinical guidelines: the Modelling Algorithm Pathways in Guidelines (MAPGuide) project.
- Enhancing the QUAlity and Transparency Of Health Research.
- Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ.
- Ethical analysis to improve decision-making on health technologies.
- Ethics and health technology assessment: handmaiden and/or critic?
- Ethics expertise for health technology assessment: a Canadian national survey
- Evidence Synthesis TSD series (NICE Decision Support Unit)
- Examining the value and quality of health economic analyses: implications of utilizing the QHES
- Exploring qualitative research synthesis: the role of patients’ perspectives in health policy design and decision-making.
- Framework for systematic identification of ethical aspects of healthcare technologies: the SBU approach.
- Future challenges for the economic evaluation of healthcare: patient preferences, risk attitudes and beyond.
- General Methods (benefit assessment) (The Institute for Quality and Efficiency in Healthcare)
- Good Practice for Budget Impact Analysis I

- Guidance for considering ethical, legal, and social issues in health technology assessment: application to genetic screening
- Guidance for developers of health research reporting guidelines.
- Healthcare technology assessment. In: Have H ten, editor. Encyclopedia of Global Bioethics. New York: Springer; 2015.
- HTA Adaptation Toolkit (EUnetHTA)
- INTEGRATE-HTA: adopting and implementing an integrated perspective on complex interventions.
- Integrating ethics in health technology assessment: many ways to Rome.
- Integration of Existing Systematic Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
- Internal validity of non-randomised studies (NRS) on interventions guidelines (EUnetHTA)
- Is there a European view on health economic evaluations? Results from a synopsis of methodological guidelines used in the EUnetHTA partner countries
- Keeping up to date with information retrieval research: Summarized Research in Information Retrieval (SuRe Info)
- Key recommendations from the MedtecHTA project.
- Mapping the integration of social and ethical issues in health technology assessment.
- Mapping to Estimate Health-State Utility from Non-Preference-Based Outcome Measures

- Measuring Drug Costs in Cost-Effectiveness Analyses: A Managed Care Perspective

- Measuring Drug Costs in Cost-Effectiveness Analyses: An Industry Perspective

- Measuring Drug Costs in Cost-Effectiveness Analyses: An International Perspective

- Measuring Drug Costs in Cost-Effectiveness Analyses: A Societal Perspective

- Measuring Drug Costs in Cost-Effectiveness Analyses: Issues and Recommendations

- Measuring Drug Costs in Cost-Effectiveness Analyses: Medicare, Medicaid, and Other United States Government Payers’ Perspectives

- Medication Compliance and Persistence: Terminology and Definitions

- Methodological guidance documents for evaluation of ethical considerations in health technology assessment: a systematic review.
- Methodology Guidelines (EUnetHTA)
- Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Methods for Integrating Medication Compliance and Persistence in Pharmacoeconomic Evaluations

- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008.
- Methods of international health technology assessment agencies for economic evaluations comparative analysis.
- Methods of synthesizing qualitative research studies for health technology assessment.
- Modeling and Simulation in the Context of Health Technology Assessment: Review of Existing Guidance, Future Research Needs, and Validity Assessment Rockville (MD): Agency for Healthcare Research and Quality (US); 2017.
- Modeling good research practices-overview

- Modelling methods for pharmacoeconomics and health technology assessment: an overview and guide.
- Multinational Trials—Recommendations on the Translations, Required Approaches to Using the Same Language in Different Countries, and the Approaches to Support Pooling the Data

- Observational Data TSD (NICE Decision Support Unit)
- Observational Evidence and Strength of Evidence Domains: Case Examples. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
- Patient-centric HTA: different strokes for different folks.
- Patient-Reported Outcome and Observer-Reported Outcome Assessment in Rare Disease Clinical Trials

- Patient-Reported Outcome Data Collection in Clinical Trials Using Mixed Modes

- PBAC Guidelines (Australian Pharmaceutical Benefits Advisory Committee)
- Pediatric Patient-Reported Outcome Instruments for Research to Support Medical Product Labeling

- Pharmacoeconomic Guidelines Around the World
- Principles of Good Practice for Budget Impact Analysis II

- Q-SEAda tool for quality assessment of ethics analyses conducted as part of health technology assessments.
- Qualitative and mixed methods provide unique contributions to outcomes research.
- Qualitative methods in patient-centered outcomes research.
- Questionnaire to Assess Relevance and Credibility of Modeling Studies for Informing Healthcare Decision Making

- Revealing and acknowledging value judgments in health technology assessment.
- Reviewing model parameters TSD (NICE Decision Support Unit)
- Risk of Bias 2 Tool
- ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions.
- Statistical Methods for the Analysis of Discrete-Choice Experiments

- STRengthening Analytical Thinking for Observational Studies. Stratos Initiative.
- Survival analysis TSD (NICE Decision Support Unit)
- Systematic Reviews of Economic Evaluations: Utility or Futility?
- Technology appraisal guidance (NICE)
- The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials.
- The EQUATOR Network and reporting guidelines: Helping to achieve high standards in reporting health research studies.
- Transferability of Economic Evaluations Across Jurisdictions

- Translation and Cultural Adaptation Process for Patient-Reported Outcomes Measures

- Use of Existing Patient-Reported Outcome Instruments and Their Modification

- Using expert opinion in health technology assessment: a guideline review.
- Validation of Electronic Systems to Collect Patient-Reported Outcome Data—Recommendations for Clinical Trial Teams

Test-ASSESSMENT
Assessment: Identifying and Interpreting Individual Studies
 | A Checklist for Medication Compliance and Persistence Studies Using Retrospective Databases |
| A Checklist for Medication Compliance and Persistence Studies Using Retrospective Databases | |
 | test |
Assessment: Interpreting Bodies of Evidence
- Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis.
- A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis.
- Assessing the Quality of Mixed Methods Research: Toward a Comprehensive Framework. In: SAGE Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: Sage Inc.; 2010
- Conducting Indirect Treatment Comparison and Network Meta-Analysis Studies

- Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews.
- Indirect Treatment Comparison/Network Meta-Analysis Study Questionnaire to Assess Relevance and Credibility to Inform Healthcare Decision Making

- Integration of Existing Systematic Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014.
- International comparison of the definition and the practical application of health technology assessment
- Interpreting Indirect Treatment Comparisons and Network Meta-Analysis for Healthcare Decision Making

- Methods for Meta-Analysis in Medical Research.
- Protocol-developing meta-ethnography reporting guidelines (eMERGe).
- RAMESES publication standards: meta-narrative reviews
- RAMESES publication standards: realist syntheses.
- The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration.
- Using qualitative evidence in decision-making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual).
CONTEXTUALIZATION
Contextualization: Deliberative Processes
- Conceptualizing and Combining Evidence for Health System Guidance. Ottawa, ON: Canadian Health Services Research Foundation; 2005.
- Deliberation as discussion. In: Elster J, editor. Deliberative Democracy. Cambridge: Cambridge University Press; 1998.
- Deliberative processes and evidence-informed decision making in healthcare: do they work and how might we know?
- Deliberative Processes in Decisions About Health Care Technologies: Combining Different Types of Evidence, Values, Algorithms and People. London: Office of Health Economics; 2009.
- Designing and Implementing Deliberative Processes for Health Technology Assessment: Joint HTAi/ISPOR Task Force Report

- Evidence-informed deliberative processes. A practical guide for HTA agencies to enhance legitimate decision-making. Version 1.0. Nijmegen, Radboud university medical centre, Radboud Institute for Health Sciences, 2019.
- Value assessment frameworks for HTA agencies: the organization of evidence-informed deliberative processes.
Contextualization: Patient Engagement and Patient Preferences
- Bringing “the public” into health technology assessment and coverage policy decisions: from principles to practice.
- Clinical Outcome Assessments: Conceptual Foundation

- Clinician-Reported Outcome Assessments of Treatment Benefit

- Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute.
- Exploring qualitative research synthesis: the role of patients’ perspectives in health policy design and decision-making.
- Guidance for patient involvement in HTA. (EUPATI)
- Incorporating the Patient's Perspective into Drug Development and Communication
- Patient and Public Involvement Policy (NICE)
- Patient-based health technology assessment: a vision of the future.
- Patient-centric HTA: different strokes for different folks.
- Patients’ perspectives in health technology assessment: a route to robust evidence and fair deliberation.
- Value to whom? The patient voice in the value discussion.
Contextualization: Weighted Stakeholder Preferences and Multi-Criteria Decision Analysis
- Advancing MCDA and HTA into Coverage Decision-Making. In: Multi-Criteria Decision Analysis to Support Healthcare Decisions. Cham, Switzerland: Springer; 2017.
- Bridging health technology assessment (HTA) with multicriteria decision analyses (MCDA): field testing of the EVIDEM framework for coverage decisions by a public payer in Canada.
- Implementation of EUnetHTA core Model® in Lombardia: the VTS framework.
- Integrating patients’ views into health technology assessment: Analytic hierarchy process (AHP) as a method to elicit patient preferences.
- Mapping to Estimate Health-State Utility from Non-Preference-Based Outcome Measures

- Multi-Criteria Decision Analysis to Support Healthcare Decisions. New York: Springer; 2017.
- Multicriteria decision analysis for including health interventions in the universal health coverage benefit package in Thailand.
- Multiple Criteria Decision Analysis for Healthcare Decision Making—An Introduction: Report 1

- Multiple Criteria Decision Analysis for Healthcare Decision Making: Report 2

- Priority setting for health technology assessment at CADTH.
- Statistical Methods for the Analysis of Discrete-Choice Experiments

- Use of Existing Patient-Reported Outcome Instruments and Their Modification

- Which health technologies should be funded? A prioritization framework based explicitly on value for money.
Contextualization: Use of Thresholds
- Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research.
- Extended cost-effectiveness analysis for health policy assessment: a tutorial.
- Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold.
- Performance-Based Risk-Sharing Arrangements—Good Practices for Design, Implementation, and Evaluation

- Systematic overview of cost-effectiveness thresholds in ten countries across four continents.
- The NICE cost-effectiveness threshold: what it is and what that means.
Contextualization: Interpreting or Adapting HTAs from Other Jurisdictions
- Challenges faced in transferring economic evaluations to middle income countries.
- EUnetHTA information management system: development and lessons learned.
- HTA Adaptation Toolkit (EUnetHTA)
- Transferability of Economic Evaluations Across Jurisdictions

- Variability of cost-effectiveness estimates for pharmaceuticals in Western Europe: lessons for inferring generalizability.
- What do international pharmacoeconomic guidelines say about economic data transferability?
Contextualization: Use of Budget Impact Analysis
IMPLEMENTATION
Implementation: Implementing HTA
- Health Systems Evidence (McMaster University)
- How can research organizations more effectively transfer research knowledge to decision makers?
- HTA Implementation Roadmap in Central and Eastern European Countries.
- Submission template for Pharmaceuticals and Submission template for Medical Devices (EUnetHTA)
- SUPPORT Tools for evidence informed health Policymaking (STP).
- Format for formulary submissions (AMCP)
- The Pharmaceutical Price Regulation Scheme. 02/07. London: Office of Fair Trading; 2007.
Implementation: Measuring HTA Impact
- Assessing the impact of health technology assessment.
- Assessing the impact of health technology assessment on the Austrian healthcare system.
- Influence Of Health Technology Assessment And Its Measurement.
- Models and applications for measuring the impact of health research: update of a systematic review for the Health Technology Assessment programme.
- Research impact: a narrative review.
- Returns on Research Funded Under the NIHR Health Technology Assessment (HTA) Programme: Economic Analysis and Case Studies.
- The impact of health technology assessment reports on decision making in Austria.
- The impact of HTA and procurement practices on the selection and prices of medical devices.
- The impact of HTA reports on health policy: a systematic review.
- What are the effects of HTA reports on the health system? Evidence from the research literature. In: Health technology assessment and health policy-making in Europe. 2008.