Background
Several years ago, ISPOR convened an ad hoc advisory body of healthcare decision makers to provide strategies to ISPOR on bridging the gap between outcomes research and healthcare decisions (Healthcare Strategy Council). Key issues identified by this advisory body were education/training, health policy/market force, methods/standards, information availability/access/sharing/applicability, and credibility/integrity/bias. ISPOR has implemented many educational programs and addresses methods/standards and credibility/integrity/bias through its Good Practices for Outcomes Research. However, information availability/applicability/access/sharing were issues which could best be addressed by professionals who use outcomes research information to develop evidence to inform healthcare decision makers. Since health technology assessment (HTA) informs many healthcare decision makers - regulatory agencies, healthcare payers, clinicians, hospitals, clinics, health authorities, and patient groups, the ISPOR Health Technology Assessment Roundtables were formed.
Mission
The mission of the ISPOR Health Technology Assessment Council is to:
- Provide a platform for regional ISPOR HTA Roundtables to share issues and recommendations
- Provide guidance in the translation and use of outcomes studies as useful information in the health technology assessment and healthcare decision-making processes.
- Address issues and concerns of decision makers globally.
- Assist in the development and implementation of initiatives related to use of outcomes research information by healthcare decision makers.
Membership and Structure
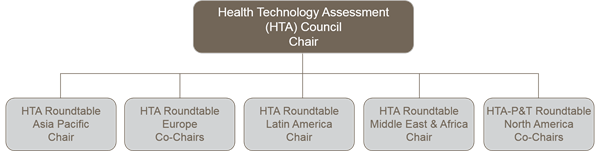
Council Chair
Jessica Daw, MBA, PharmD
Asia Pacific Roundtable Chair
Europe Roundtable Co-Chairs
Latin America and the Caribbean Roundtable Chair
Middle East and Africa Roundtable Chair
North America Co-Chairs
Publications & Presentations
For More Information
For more information about health technology assessment, please see HTA Strategic Initiatives or HEOR by Topic: HTA.
Please contact us for information about the Health Technology Assessment Council, Roundtables, or other HTA initiatives.
