Yoko Ishiguro, MPP. Reimbursement Manager of Health Care Economics & Government Affairs, Medtronic North Asia (Korea and Japan). yoko.ishiguro@medtronic.com
Micra AV selection for HTA
In April 2019, Japan HTA scheme known as cost-effectiveness evaluation (CEE) was introduced as a new repricing (price adjustment) methodology. It covers both drug and medical technology with same selection criteria, analysis guideline and operation scheme.
While several drugs were selected for CEE scheme, none from MedTech industry were selected until October 13, 2021. On that day, Micra AV (Medtronic), the first dual-chamber leadless pacemaker, was selected for CEE.
A leadless pacemaker is a capsule-sized implantable electronic device placed inside of heart via delivery system, while a conventional pacemaker is placed in a pocket made in patient’s chest with leads running through the heart. Micra VR, the first
leadless pacemaker (Medtronic) launched on September 1st, 2017, is well known for less invasiveness, but the indication is generally limited to patient eligible for a single chamber pacemaker (1). The device called Micra AV (Medtronic) is in same
size, weight, and procedure as Micra VR though it has a feature as a dual chamber pacemaker. Micra AV received reimbursement approval from Central Social Insurance Medical Council (CSIMC, locally known as Chuikyo) on October 13 with below conditions
(2).
- Price: JPN 1,170,000
- Pricing methodology: Similar functional category pricing methodology
- Rational: 10% premium over Micra VR (JPN 1,060,000)
- Definition of new functional category
- Micra AV has the new mechanism for efficacy which is significantly different from conventional technology
- Expected peak sales in 10-year:
- JPN 7.6 billion at 10 year from launch
Because expected peak sales were more than the threshold described in the CEE selection guideline, Micra AV was selected for CEE based on H2 category. This is the first medical technology to be affected by CEE requirement in MedTech industry. Prior to
this selection, MedTech industry presented its opinion to the government that medical technology requires more careful evaluation than drug due to distinct attributes such as lack of randomized controlled trials (RCTs), small sample size in clinical
trials, short life cycle, operator’s learning curve. Multiple benefits of medical technology are not fully recognized by incremental cost effectiveness ratio (ICER) (3,4).
CEE Amendment plan next April
Chuikyo members discussed the amendment plan proposed by Ministry of Health, Labor, and Welfare (MHLW) on October 15. MHLW proposed the plan about price adjustment methodology change and structure improvement. This is the second Chuikyo meeting about
the topic; in the first one, MHLW made a proposal on operation scheme change. The main topic was price adjustment methodology based on analysis results. An objective of Japan CEE is not reimbursement determination as other countries’ HTA but
price adjustment after initial reimbursement decision-making. The price adjustment scope is the portion of uplift amount (premium price) that was additionally granted to a new medical technology compared to conventional ones. For example, a price
adjustment target for Micra AV is its price difference from Micra VR (JPY 1,170,000-1,060,000=110,000). Once ICER is determined in the CEE process, the specific “correction coefficient” based on ICER value will be adapted to the uplift
amount. The rule of correction coefficient based on ICER has been fixed but some of the cases couldn’t show ICER because cost minimization analysis (CMA) was adapted. The proposal from MHLW is to set the rule for correction coefficient for a
case in CMA. Originally, there was no rule, but as MHLW decided to use the minimum correction coefficient to existing cases, it was then set as a rule. MHLW is concerned about CEE document submission delays as they could lead to delay in price cuts.
MHLW also proposed to set a rule for a case in which a CEE report is submitted after the deadline (9-month from product selection). The first step is manufacturer’s explanation on the reason for failing to meet the deadline. If the rationale
is unreasonable, the minimum correction coefficient in the current rule (Table 1) is adapted. Chuikyo members accepted the amendment plan.
Table 1: Analysis result and correction coefficient (1)
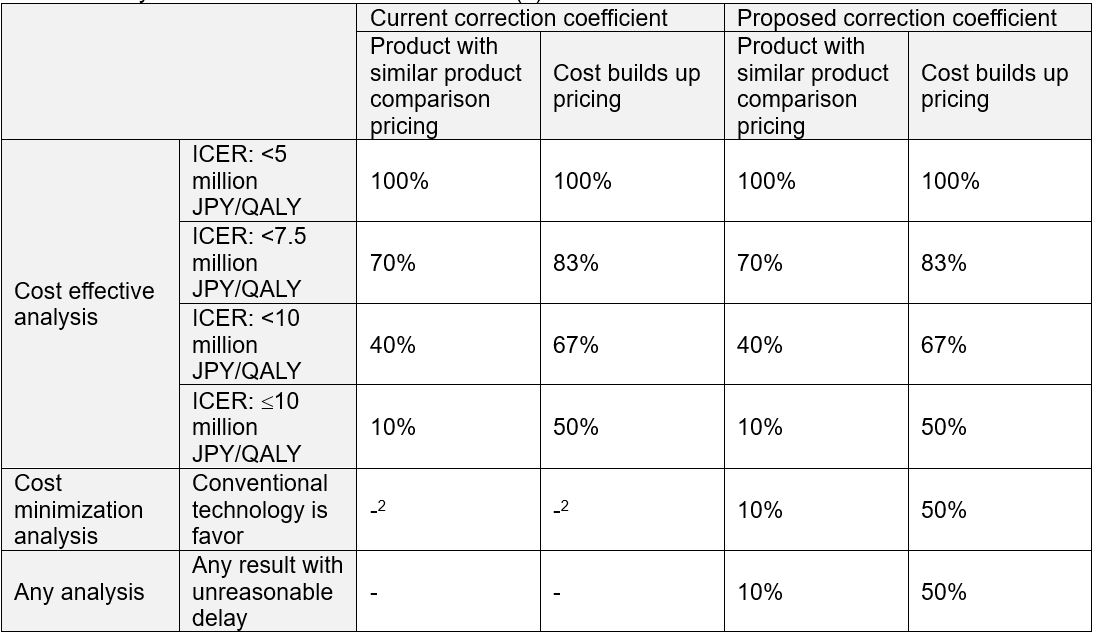
1 This table shows the basic rule. There is another table for orphan technology
2 There is no rule but MHLW decided to adapt the minimum correction coefficient in the current rule to existing cases
References
- Nogami, Akihiko et al. “JCS/JHRS 2021 Guideline Focused Update on Non-Pharmacotherapy of Cardiac Arrhythmias. (https://www.j-circ.or.jp/cms/wp-content/uploads/2021/03/JCS2021_Kurita_Nogami.pdf)”
- Chuikyo meeting transactions of 13th October (https://www.mhlw.go.jp/content/12404000/000842515.pdf)
- Chuikyo meeting transactions of 4th August (https://www.mhlw.go.jp/content/12404000/000816093.pdf)
- https://press.ispor.org/asia/index.php/2021/08/18/japan-update-industry-groups-ask-for-no-major-change-in-japan-hta-reform/