Regulatory Pathways in Europe, the United States, and Japan and Health Technology Assessments for Gene Therapies
Yitong Wang, PhD, Aix-Marseille University, Marseille, France; Tingting Qiu, PhD, Aix-Marseille University, Marseille, France; Shuyao Liang, PhD, Aix-Marseille University, Marseille, France; Claude Dussart, PhD, Université Claude Bernard Lyon 1, Lyon, France
Over the past few years, several gene therapies have been approved and adopted in many countries and a substantial number of them are in the pipeline.1 The US Food and Drug Administration (FDA) is expecting to receive more than 200 investigational new drug applications per year, and, by 2025, to approve 10 to 20 cell and gene therapies per year.2 However, considering the uncertainty of long-term clinical evidence and the high prices associated with gene therapies, transitioning such new advances from the bench to the bedside is often challenging.3,4 This paper provides an overview of cell and gene therapies, marketing-authorization pathways in the European Union (EU), the United States, and Japan. Additionally, the regulatory and reimbursement status of gene therapies were compared in the United States and 5 European countries: France, the United Kingdom (England and Scotland), Germany, Italy, and Spain.
Marketing Authorization Pathways and Expedited Approval Programs
Authorities in Europe, the United States, and Japan have developed various expedited approval programs (Table 1). These adaptive regulatory pathways aim to accelerate the market approval of gene therapies and vary to a great degree between the 3 authorities.
Table 1. The marketing authorization regulation agencies and Expedited programs in Europe, the United States, and Japan
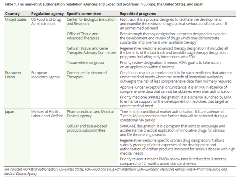
In the European Union, gene therapies (defined as advanced therapy medicinal products [ATMPs]) are regulated like other pharmaceuticals through a centralized marketing authorization procedure to ensure a single evaluation and authorization decision applicable to all EU countries (Figure 1). The Committee for Advanced Therapies assesses the quality, safety, and efficacy of gene therapies based on the marketing authorization application submitted by the manufacturers and prepares a draft opinion on the application. The Committee for Medicinal Products for Human Use reviews the recommendations from the Committee for Advanced Therapies and adopts a final opinion on the marketing authorization decision. Apart from the standard marketing authorization pathway, conditional marketing authorization and marketing authorization under exceptional circumstances are also established. Gene therapies are eligible for conditional marketing authorization if they have a positive benefit-risk balance and are likely to satisfy unmet medical needs. The marketing authorization holders must fulfill the scientific obligations to submit the postmarket confirmatory evidence before the conditional marketing authorization may be converted into a standard marketing authorization. Unlike the conditional marketing authorization, the marketing authorization under exceptional circumstances is granted when comprehensive data could not be possible to generate due to the disease rarity or unethical considerations.
Figure. Gene therapies, regulatory pathways in Europe and Japan
.tmb-medium.jpg?Culture=en&sfvrsn=df7278af_1)
In Japan, gene therapies (classified as regenerative medicines) are regulated by the Ministry of Health, Labour, and Welfare (MHLW) under a specific, unique, fast-track approval framework different from all other pharmaceuticals (Figure 1). The new legislative framework was proposed by the Act on the Safety of Regenerative Medicine (RM Act) and the Pharmaceuticals and Medical Devices Act (PMD Act) in November 2013, aiming at expediting the development and marketing authorization of regenerative medicines in Japan. A conditional time-limited marketing authorization may be issued by the Pharmaceuticals and Medical Devices Agency (PMDA) based on preliminary clinical trials indicating likely efficacy and confirmed safety. However, the marketing authorization holders must submit a second application to the PMDA within a pre-defined period (with a maximum of 7 years), in order to reassess whether the regenerative medicines meet the requirements for standard marketing authorization based on postmarketing evidence. In Japan, for products targeting serious or life-threatening diseases without effective or available treatment, “SAKIGAKE” designation is proposed by the MHLW to encourage industry involvement in innovative products and to promote the marketing authorization ahead of other countries. The SAKIGAKE-designated drugs also benefit from prioritized consultation, accelerated review time, extended re-examination period, and premium pricing.
In the United States, gene therapies are classified under human cells, tissues, and cellular- and tissue-based products (HCT/P’s) and are regulated as drugs and/or biological products by the Center for Biologics Evaluation and Research. A conditional approval pathway similar to the fast-track framework in Japan, was proposed in the REGROW Act by the FDA in March 2016, which sought to eliminate phase III studies to create an expedited approval pathway accepting less comprehensive evidence of safety and effectiveness. However, the REGROW Act was opposed by the academic community out of concern that the lower standards for marketing authorization would allow ineffective and possibly unsafe products to reach the market. A new accelerated approval pathway for regenerative medicines, known as Regenerative Medicine Advanced Therapy (RMAT) designation, was introduced in the 21st Century Cures Act in December 2016, with the intention to incentivize the development and marketing authorization of regenerative medicines addressing unmet medical needs for serious or life-threatening disease. An additional 4 expedited programs for drugs that target to serious diseases especially without available treatments exist in the United States, including the fast-track designation, breakthrough therapy designation, accelerated approval, and priority review designation.
HTAs for Gene Therapies
To date, 7 gene therapies were approved in the European Union and/or the United States, among which alipogene tiparvovec (Glybera) marketing authorization was withdrawn by the developing company due to commercial reasons (Table 2).5,6 The HTA decisions for gene therapies from 5 European countries are summarized in Table 3. Unlike many countries with official HTA agencies, the United States does not have a national and formal HTA body to evaluate the technologies and make recommendations for reimbursement and pricing. The Institute for Clinical and Economic Review (ICER), founded in 2006, was an independent nonprofit organization to evaluate the clinical and economic value of the technologies, which has become very popular and influential on the United States healthcare system.
Table 2. Marketing authorization of gene therapy in European Union and the United States
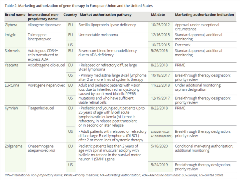
In the United States, ICER recognized that tisagenlecleucel (Kymriah), axicabtagene ciloleucel (Yescarta), and voretigene neparvovec (Luxturna) offered a net health benefit despite uncertainty related to their evidence. ICER considered tisagenlecleucel and axicabtagene ciloleucel cost-effective within the context of a threshold of $150,000 per quality-adjusted life year (QALY) gained in the United States. These therapies seemed to be priced in alignment with clinical benefits over a lifetime time horizon according to ICER’s evaluation.7
Table 3. Marketing authorization of gene therapy in European Union and the United States
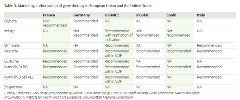
In France,8 3 gene therapies, axicabtagene ciloleucel (Yescarta), voretigene neparvovec (Luxturna), and tisagenlecleucel (Kymriah), were recommended for reimbursement by the French National Authority for Health (HAS), which were all considered to demonstrate an important actual clinical benefit (Service medical rendu [SMR]: important). Alipogene tiparvovec (Glybera) was not recommended by the HAS and was considered to have insufficient medical benefit due to heterogeneity of effectiveness, uncertainty of safety, and limitations of methodology.
In Germany,9 only talimogene laherparepvec (Imlygic) was attributed to have no added benefit due to the use of inappropriate comparator from the Federal Joint Committee’s perspective. Three gene therapies, alipogene tiparvovec (Glybera), tisagenlecleucel (Kymriah), and axicabtagene ciloleucel (Yescarta), were granted non-quantifiable additional benefit. A fourth gene therapy, voretigene neparvovec (Luxturna), was recognized as providing a considerable added benefit. All 4 gene therapies, recommended by the German Institute for Quality and Efficiency in Health Care, are orphan drugs, which are automatically granted an additional benefit by law irrespective of the available clinical evidence.
In England,10 the National Institute for Health and Care Excellence (NICE) has recommended the reimbursement of talimogene laherparepvec (Imlygic) in the restricted subgroup population with best responses. Tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) were approved for use within the Cancer Drugs Fund while further data collection is ongoing. Autologous CD34+ cells transduced to express ADA (Strimvelis) was recommended by NICE for its clinical benefits in improving survival and its cost–effectiveness below the threshold for highly specialized technologies at £100,000 per QALY gained. In Scotland,11 tisagenlecleucel for 2 indications and axicabtagene ciloleucel were recommended for reimbursement by the Scottish Medicine Consortium with patient access schemes.
Three gene therapies, axicabtagene ciloleucel (Yescarta), voretigene neparvovec (Luxturna), and tisagenlecleucel (Kymriah) were recommended by the Italian Medicines Agency12 in Italy with managed entry agreements. Two gene therapies, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel, were recommended to be used in authorized centers in Spain.13
Translation Insights
Substantial efforts have been made by regulators to accelerate the assessment and approval of transformative gene therapies.14 Variations still exist in marketing authorization pathways and expedited approval programs for gene therapies across the European Union, the United States, and Japan due to the different regulatory environments and public health needs. Enhanced international coordination is recommended to standardize the marketing authorization requirements, in order to minimize duplicated work and to facilitate the availability of gene therapies globally.
After marketing authorization, patient access to gene therapies ultimately depends on decisions made by payers and HTA organizations. Payers need to maintain a balance between ensuring access to medical innovation and encouraging sustainable development of cell and gene therapy.15 Various approaches have been adopted by different countries to mitigate the potential risk of reimbursing gene therapies with substantial uncertainties surrounding long-term outcomes.16 Payers generally have expressed openness to such innovation. However, it is questionable how they will react and deal with the continuously increasing number of gene therapies seeking market access.
References
1. Qiu T, Dabbous M, Borislav B, Toumi M, Hanna E, Health technology assessment of gene therapies in Europe and the USA: Analysis and future considerations. Cell Gene Ther Insights. 2019;5:1043-1059.
2. Statement from FDA Commissioner Scott Gottlieb, MD and Peter Marks, MD, PhD, Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. US Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics. Accessed June 28, 2020.
3. Ilic N, Savic S, Siegel E, Atkinson K, Tasic L. Examination of the regulatory frameworks applicable to biologic drugs (including stem cells and their progeny) in Europe, the U.S., and Australia: part I–a method of manual documentary analysis. Stem Cells Transl Med. 2012;1(12):898-908.
4. Witten CM, McFarland RD, Simek SL, Concise Review: The US Food and Drug Administration and regenerative medicine. Stem Cells Transl Med. 2015;4(12):1495-1499.
5. Approved cellular and gene therapy products. US Food and Drug Administration https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products. Accessed July 24, 2020.
6. European Medicines Agency. https://www.ema.europa.eu/en. Accessed June 20, 2020.
7. Institute for Clinical and Economic Review report finds costs of approved CAR-T therapies align with clinical benefit. https://icer.org/pressreleases/car-t-evidence-report/. Accessed June 20, 2020.
8. French health authority. https://www.has-sante.fr/portail/. Accessed June 20, 2020.
9. Institute for Quality and Efficiency in Health Care. https://www.iqwig.de/en/home.2724.html. Accessed June 20, 2020.
10. The National Institute for Health and Care Excellence. https://www.nice.org.uk/. Accessed June 20, 2020.
11. Scottish Medicine Consortium. https://www.scottishmedicines.org.uk/. Accessed June 20, 2020.
12. The Italian Medicines Agency. https://www.aifa.gov.it/. Accessed June 20, 2020.
13. Agencia de Evaluación de Tecnologias Sanitarias. http://www.isciii.es/. Accessed June 20, 2020.
14. FDA. FDA’s Efforts to Advance the Development of Gene Therapy. https://www.fda.gov/news-events/fda-voices/fdas-efforts-advance-development-gene-therapy. Accessed June 20, 2020.
15. Barlow JF, Yang M, Teagarden JR. Are payers ready, willing, and able to provide access to new durable gene therapies? Value Health. 2019;22(6):642-647.
16. Hanna E, Toumi M, Dussart C, et al. Funding breakthrough therapies: A systematic review and recommendation. Health Policy. 2018;122(3):217-229.
Explore Related HEOR by Topic

