Sandhu G 1, Izzuna MMG 2, Li SC 3, Kilburg A 4 & Lee KKC 5
1Gurmit Sandhu, B Pharm (Hons), MBA, MPH, Gurmit Sandhu Consulting GmbH, Basel, Switzerland
2 Izzuna Mudla Mohamed Ghazali, MBBS, MPH, Medical Development Division Ministry of Health Malaysia, Malaysian Health Technology Assessment Section (MaHTAS), Putrajaya, Malaysia
3 Shu Chuen Li, MApplSc, MBA, PhD, School of Biomedical Sciences and Pharmacy,
Faculty of Health and Medicine, University of Newcastle, Callaghan, NSW, Australia
4 Anne Kilburg, MSc, KilburgDialogue, Allschwil, Switzerland
5 Kenneth Kwing Chin Lee, MPhil, PhD, School of Pharmacy, Faculty of Health & Medical Services, Taylor`s University, Subang Jaya, Malaysia
Corresponding author: Gurmit Sandhu, gurmitsandhu97@hotmail.com
Introduction
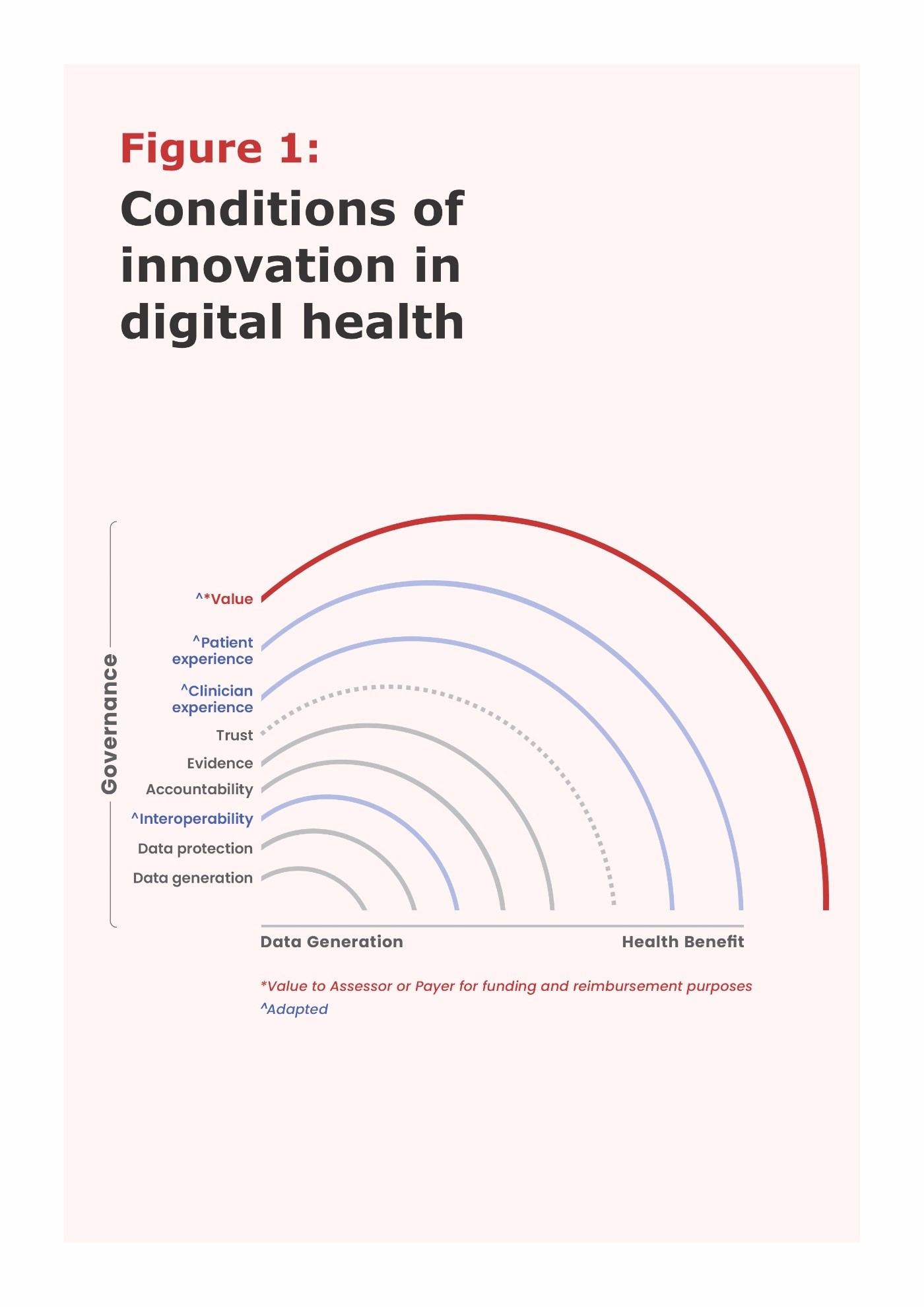
Innovation conditions for Digital Health Technologies (DHTs) can be illustratively described along the continuum from data generation to health impact including data access, interoperability, data protection, accountability, evidence, trust, patient & clinician experience and then value to assessors and ultimately, to society (Figure 1. Adapted by Sandhu G & Kilburg A, personal communications 2022). All these conditions can be described as part of health data governance 1.
The term “digital health” is rooted in eHealth, which is defined as “the use of information and communications technology ICT in support of health and health-related fields” 2. There are many subsets including mHealth, Remote Patient Monitoring, Internet of Things and Telemedicine. More recently, the term “digital health” was introduced as “a broad umbrella term encompassing eHealth, as well as emerging areas, such as the use of advanced computing sciences in ‘big data’, genomics and artificial intelligence (AI)” 2. DHTs have been pledged as an enabler of sustainable health systems but have yet to demonstrate their potential at scale & speed.
Users of DHTs are diverse including patients/citizens, healthcare providers, health care systems and data services 2. These can be patient - or clinician-facing DHTs. It is pertinent to differentiate between users from primary & secondary beneficiaries especially when patients are the users since they may not always be primary beneficiaries.
Significant differences exist in the purpose of DHTs between Low- and Middle-Income Countries (LMICs) and High-Income Countries (HICs): while in LIMCs, the focus is on healthcare system strengthening, in HICs it is more on proactively offering diagnosis and treatment. In LMICs, incentives for assessing and adopting DHTs vary. Other considerations include the lack of affordability, societal & cultural beliefs that are against DHTs and their usage, along with large variations in socio-economic factors especially urban vs. rural settings make the adoption of DHTs in LMICs challenging.
Despite these differences in different economic conditions and purpose of usage, the common element across these countries is “data” where the WHO has declared that it shall treat data as a public good 3, thereby creating unfamiliar opportunities & challenges for HTA assessors and patients.
For example, the DHT focus on the Malaysian Strategic Plan for Cancer Control 4 is on digital pathology and radiology to help increase screening coverage, early diagnosis, and services. A priority area includes establishing a national cancer registry to collect Real Word Data on cancer care/management and health outcomes.
Challenges for the use of DHTs such as telemedicine which is available in most LMICs are listed in Table 1 (adapted from Yadv et al 2021 5).

Challenges in Assessment & Adoption of DHTs
“Mistrust” in DHTs often stems from knowledge gaps in these technologies, poor Usability & Accessibility for patients and/or healthcare professionals (HCPs), and unresolved ethical concerns. For example, drivers of “mistrust” in DHTs in Malaysia include (a) stringent regulatory requirements to fulfil (b) lack of reimbursement and funding especially from public payers/funders, where often there is a significant out of pocket costs to patients for these DHTs. (c) their adoption slower than expected, perpetuating the “mistrust”
Despite these challenges, the COVID-19 pandemic spearheaded the improvement of digital literacy across a range of stakeholders either as professionals and/or citizens. Innovative national government policies on the broader digital Malaysian economy were instrumental to accelerate the adoption of DHTs. Better connectivity across urban & rural Malaysia and improved technologies also contributed to this acceleration.
From a HTA perspective, the main challenges along with suggestions to address these, in the assessment and adoption of DHTs in Malaysia include:
(a) Lack of sufficient evidence. Technologists & manufactures of DHTs are encouraged to directly understand the evidence requirements and plan early for this.
(b) Define the expected users and target populations of the DHT, which may be different on how technology developers define these. In addition, characterise the infrastructure readiness where the DHT will be deployed. Describe the health and digital literacy of the user & their beneficiaries. Published Digital Readiness surveys in healthcare can be a helpful starting place. Use this opportunity to collaborate with a medical professional society and/or patient groups to understand their digital literary status and how they would like to improve these.
(c) Provide & address Clinical Information Quality 6 expectations.
In addition to their Usability & Availability of data from DHTs, HCPs have informativeness requirements which includes accuracy, completeness, interpretability, plausibility, trustworthiness & relevance of outputs from DHTs to the specific clinical situation of their patients.
(d) Funding for addressing short term issues such as interoperability alignment to enable use of a DHT and longer-term maintenance of these DHTs.
Key Success Factors for implementation of DHTs
In Australia, a successful digital health implementation has included national electronic patient records, telehealth and e-prescriptions. These were a result of the Australian Digital Health Strategy (2018 -2022) initiative with several focus areas: (a) interoperability & access to health data, (b) data security, (c) data quality, (d) workforce preparation, and (e) public & citizen engagement. Implementation was managed by the Australian Digital Health Agency.
Similarly, the Singaporean Ministry of Health launched three strategic and innovative DHT policies: (a) National Electronic Health Records in 2011 (b) National AI strategy with one of the focuses on health in 2019, and (c) Licensed telemedicine in 2022 for health care providers in private & public settings. Support is now being provided how to customise medical devices to Singaporean requirements for their usage and how they are being developed. Both as HICs along with Malaysia (an upper middle-income country), they had innovative and broader governmental policies & implementation on overall national digital transformation, along with a focus on the healthcare sector. There was ongoing co - ordination across government agencies responsible for healthcare and other areas.
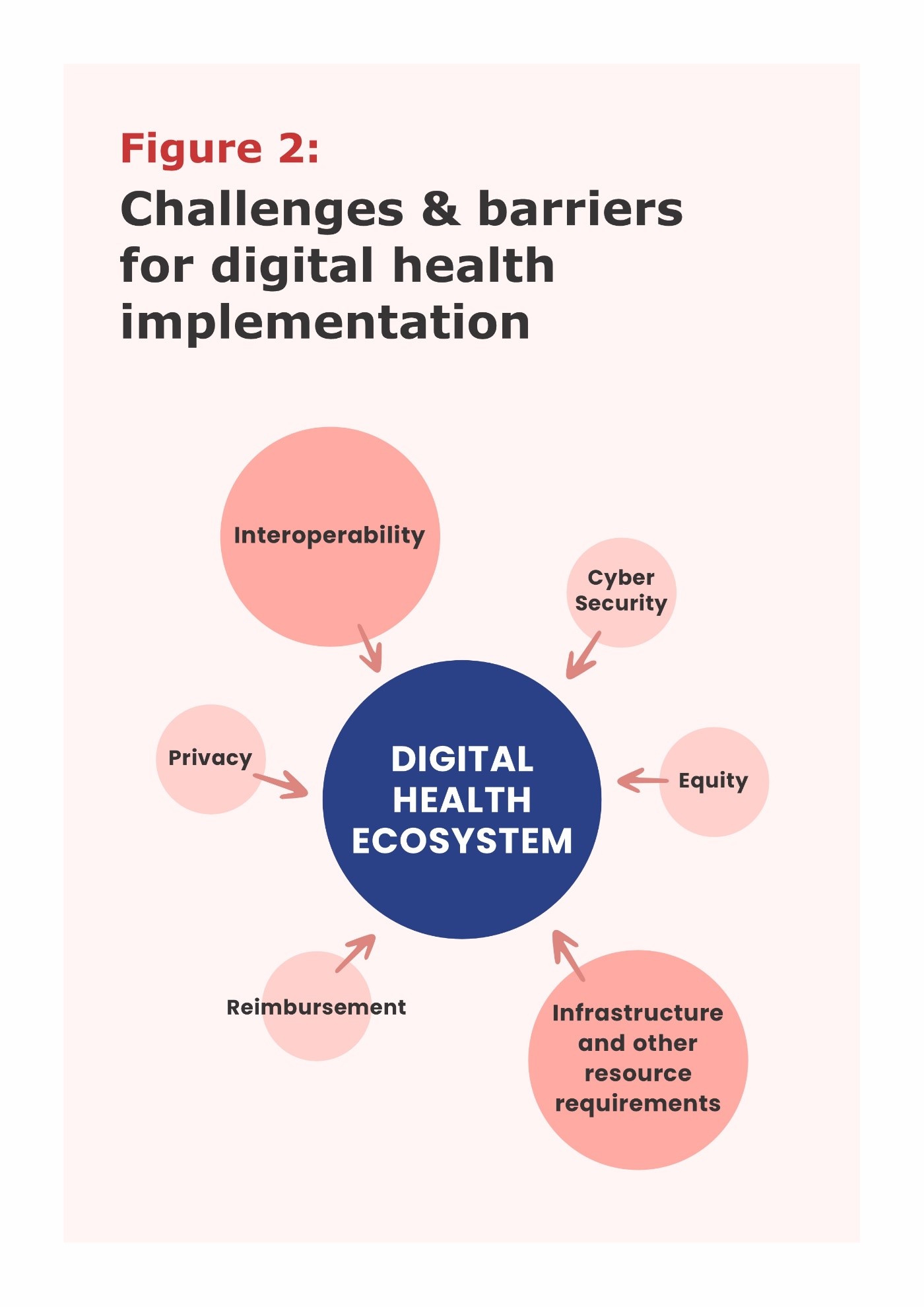
Figure 2 describes the challenges & barriers for Digital Health Implementation in HICs (e.g., Australia & Singapore). Interoperability with existing healthcare systems prevails as a key challenge. Cybersecurity risks, breaches of data privacy and imbalance of equity in receiving care are also important barriers. Despite being HICs with successful DHT implementation, a lack of comprehensive (direct & indirect considerations) and feasible criteria for reimbursement of DHTs remains a barrier. Long-term sustainability of DHTs should also be considered as part of the reimbursement criteria.
Patients Perspectives on “Mistrust” in DHTs
“Trust” as a “gut feeling” is shaped by many factors and is at the core of the relationship and communications between patients and their HCPs (Figure 1). Data protection, accountability (roles and responsibilities) and evidence supporting the value of DHTs can strongly influence the foundation of “Trust”. Poor or no patient and clinician experiences with DHTs do not help to reduce the “Mistrust” about DHTs.
With the rapid use of AI algorithms for clinical decision support in DHTs, the notion of trustworthiness of these outputs is embedded around “Explainable AI” (Figure 3. Adapted from Lötsch et al 7). There are five components (Figure 3) of trustworthiness including informativeness especially on the quality of data and evidence which is the basis for HCPs in their evaluation of a particular DHT. Some scholars have proposed the concept of “minimally acceptable explainability” as a basis for sufficient understanding for HCPs & Patients communications on AI algorithms 8.

Ethical concerns include stigmatisation and marginalisation, lack of involvement in clinical decision-making, burden of data collection, risk of bias, loss of autonomy, lack of accountability on data security and privacy, and the rise of “ethics washing”.
Outlook & Summary
During the ISPOR Asia Pacific Issues Panel 2022 9, an audience poll suggested that in LMICs, most attendees preferred “minimum local evidence standards” over “best practice international standards” when determining the value of DHTs. While this is an attractive option, currently there are no published local evidence standards in LMICs. Not surprisingly, when polled, attendees mostly preferred Real-World Data in the absence of randomised controlled trials as acceptable data sources to evaluate evidence for DHTs in LMICs, such as using data from medical records as Real-World Evidence (RWE) for regulatory approval. Examples are available from the USA 10, such as radiological computer aided triage and notification software, ContaCT. More such case studies from LMICs need to be shared to help technologists and DHT developers to plan for their evidence, along with suppliers of electronic health records and administrators of patient registers.
In summary, DHTs will be a key component of healthcare delivery in the future. However, in LMICs considerable gaps and challenges are likely to persist especially on affordability, accountability, societal & cultural beliefs. There is an urgent need to engage and connect all stakeholders such as patients, clinicians, assessors, payers, funders, administrators of healthcare institutions, regulators, healthcare policy makers, data ethicists, DHT developers & technologists for LMICs healthcare systems to excel – especially, for improvement in quality of care and expand patient access to care. More educational efforts are required for assessors to appreciate the importance of ethical aspects associated with & technical product information/ use of DHTs. Encouragingly, usability & accessibility of DHTs are now of high importance along with the effectiveness of DHTs.
References
- Vayena E, Haeusermann T, Adjekum A, Blasimme A. Digital health: meeting the ethical and policy challenges. Swiss Medical Weekly. 2018;148: w14571. doi:10.3929/ethz-b-000239873.
- WHO guideline: recommendations on digital interventions for health system strengthening. Geneva: World Health Organisation; 2019. www.who.int. Accessed 22 December 2022. https://www.who.int/publications/i/item/9789241550505
- Ethics and governance of artificial intelligence for health. Geneva: World Health Organisation; 2021. www.who.int. https://www.who.int/publications/i/item/9789240029200.
Accessed 22 December 2022.
Ministry of Health Malaysia. Kuala Lumpur: 2021. https://www.moh.gov.my/moh/resources/Penerbitan/Rujukan/NCD/Kanser/National_Strategic_Plan_for_Cancer_Control_Programme_2021-2025.pdf. Accessed 22 December 2022.
Yadav K, Ginsburg O, Basu P, Mehrotra R. Telemedicine and cancer care in Low- and Middle-Income Countries during the SARS-CoV-2 pandemic. JCO Global Oncology. 2021;(7):1633-1638. doi:10.1200/go.21.00249
Fadahunsi KP, Wark PA, Mastellos N, Gallagher J, Majeed A, Car J. Clinical information quality of digital health technologies: protocol for an international eDelphi study. BMJ Open. 2022;12(4):e057430. doi:10.1136/bmjopen-2021-057430
Lötsch J, Kringel D, Ultsch A. Explainable Artificial Intelligence (XAI) in biomedicine: Making AI decisions trustworthy for physicians and patients. BioMedInformatics. 2021;2(1):1-17. doi:10.3390/biomedinformatics2010001
Arbelaez Ossa L, Starke G, Lorenzini G, Vogt JE, Shaw DM, Elger BS. Re-focusing explainability in medicine. DIGITAL HEALTH. 2022; 8:205520762210744. doi:10.1177/2055207622107448
Lee KKC, Li SC, Izzuna MMG & Sandhu G. Can we do better for health technology assessors & Patients? How to overcome “Mistrust” in digital technology for Asia Pacific? ISPOR Asia Pacific Issues Panel Sep 2022
- USA FDA Centre for Devices and Radiological Health CDRH. Use of real-world evidence to support regulatory decision-making for medical devices. U.S. Food and Drug Administration. Published August 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices Accessed 22 December 2022.