Early Health Technology Assessment Advice: Opportunities to Achieve Collaborative and Efficient Dialogue
Flora Wu, BA, Analyst, CBPartners, New York, NY, USA; Kay Rusher, BS, Manager, CBPartners, New York, NY, USA; Maximilian Hunt, BS, Senior Principal, CBPartners, New York, NY, USA
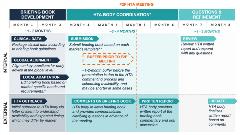
Early health technology assessment (HTA) advice, also called scientific advice, is a formal process that allows manufacturers to gain nonbinding feedback from HTA bodies on clinical trials prior to the launch of a product or device (Figure 1). To submit an early HTA advice request, manufacturers must prepare and submit a briefing book, which requires substantial time and internal coordination to align on key questions and position statements. Briefing book requirements include aspects such as disease background and current treatments, product information, and the planned study design.
Figure 1. Considerations for Manufacturers Seeking Early HTA Advice
What Is Early HTA Advice?
Early HTA advice varies because it is available at the national, HTA and regulatory, or multi-HTA level. At the national level, European Union (EU) countries such as France, Germany, and the United Kingdom offer formal national procedures for market-specific advice. At the HTA and regulatory levels, parallel consultations with the European Medicines Agency and HTA bodies offer joint regulatory and HTA advice. At the pan-EU level, the European Network for Health Technology Assessment (EUnetHTA) multi-HTA early dialogues allow manufacturers to get feedback from more than one market.
Early HTA advice also varies by market in terms of types of advice offered, product requirements, and advice fees. The UK’s National Institute for Health and Care Excellence (NICE), the French Authority of Health (HAS), and Germany’s Federal Joint Committee (G-BA) all have formal national HTA advice, joint regulatory advice, and EUnetHTA advice offerings. NICE and the G-BA also offer joint advice with local regulatory agencies (ie, Medicines and Healthcare Products Regulatory Agency, Federal Institute for Drugs and Medical Devices, Federal Institute for Vaccines and Biomedicines). NICE also offers joint advice with Canadian Agency for Drugs and Technologies Health (CADTH) and can advise on modeling questions (ie, PRIMA [Preliminary Independent Model Advice]). At the current time, NICE offers the most customizable option with higher fees, whereas HAS requirements are stricter, but advice is free.
Benefits of Seeking Early Advice as a Manufacturer
By seeking early advice, manufacturers can gain deeper insight into market-specific HTA requirements and how these may differ based on the product situation. Early advice requires internal manufacturer coordination between global, regional, and local teams to assist in the development of a global briefing book for submission. The manufacturer will also gain specific feedback on trial design-related aspects such as patient population, comparator, and endpoint selection, which ensures that if feedback is implemented, the phase III trials are appropriately designed to meet HTA expectations. This feedback provides expectations for the future evaluation of a product that can help manufacturers understand key potential HTA drivers and detractors to help strengthen the future dossier. Finally, engaging as early as possible with HTA bodies highlights an openness to collaboration.
On two recent occasions, early HTA advice has proven to be beneficial for product assessment. In the case of Novartis’ Luxturna, the gene therapy benefited from early dialogue with NICE to secure their authorization in record time of 20 weeks (compared with the average of 38 weeks). In the first meeting with NICE’s evaluation team, clinical efficacy, cost-effectiveness, patient need, and service requirements were discussed. According to Meindert Boysen, director of the Center for Health Technology Evaluation at NICE, “The company’s willingness to work with [NICE] early and constructively has allowed [NICE] to publish guidance on a much faster timeline than normal which is good news for patients.” The evaluation process was streamlined, requiring less time to reach the final evaluation determination stage because neither a draft consultation or a second committee meeting was held.1,2
"Timing is key for ensuring a successful advice process, as manufacturers must determine the point where enough alignment is reached in the clinical development plan, but with enough time to act on the advice prelaunch."
Novartis sought advice from NICE on early evidence generation for their collaboration with Innovative Medicines Initiative project PREFER, a preference study with chronic obstructive pulmonary disease (COPD) patients. The project aims to understand how patients cope with clinical burden associated with different COPD symptoms. Nigel Cook, head of Decision Support & Insights of Global Patient Access at Novartis, acknowledged that the early advice will “improve the design of the COPD patient preference study. Collecting certain outcome data alongside the patient preferences, one of the advice recommendations, will also help in correlating the preference results with current NICE processes for evaluating new treatments.”3,4 The feedback helped transform the study design and improve the quality of current processes.
Benefits of Offering Early Advice as a Payer
It is advantageous for both the HTA body and manufacturer to level-set expectations and achieve alignment as early as possible. A stronger future working relationship with the manufacturer can be established if advice recommendations are acted on. Payers will also gain an increased awareness of pipeline therapies and understand how the treatment paradigm may evolve in the next few years. Not only are there benefits to the payer in their dialogue with the manufacturer, payers can also gain perspectives from other payer stakeholders in joint or parallel consultations. In collaborative consultations, payer stakeholders can streamline their advice through a single process. For example, in response to NICE and CADTH’s collaboration to offer parallel scientific advice, Jeannette Kusel, director of NICE Scientific Advice, recognized that the “new collaboration with CADTH uses the synergies between the English and Canadian systems and provides companies with comprehensive and practical advice from both countries through a single, streamlined process.”5
The Importance of Timing to Ensure a Successful Advice Process
Timing is key for ensuring a successful advice process, as manufacturers must determine the point where enough alignment is reached in the clinical development plan, but with enough time to act on the advice prelaunch. Early advice can be incorporated into the pivotal trial design-planning process, although if pursued too early, there may be internal disagreements on major trial design aspects. The window of opportunity typically occurs after the phase II trials have been conducted, but before the phase III trials have started. While abbreviated advice processes are available in some markets, such as NICE,6 a standard advice process can take around 6 to 8 months (Figure 2) due to the need to coordinate internally and externally.7,8
Figure 2. Timeline overview of the early HTA advice
Considerations for Manufacturers to Make the Most of Early HTA Advice
It is important that the manufacturer selects the appropriate type of advice, depending on manufacturer capabilities and markets of interest. By developing a global briefing book, local affiliates can pursue early advice on their market efficiently without exhausting local resources. Local team involvement is key, and briefing book materials typically need to be submitted in the local language. Within the briefing book, strong question development should be considered because early advice is only as beneficial as the questions that are asked. Additional expert input via market research can help refine the appropriate access questions. While early advice may resolve remaining strategic questions after a product’s access challenges and opportunities are well understood, it is not a means to identify these challenges and opportunities. Overall, both manufacturers and payers can work towards a streamlined process and seek early HTA and regulatory advice to help lower barriers to patient access. •
References
1. Schofield I. Novartis’s Early HTA Engagement Pays Off with Luxturna. Pink Sheet, Informa, Sept 6, 2019. https://pink.pharmaintelligence.informa.com/PS140798/Novartiss-Early-HTA-Engagement-Pays-Off-With-Luxturna. Accessed September 17, 2020.
2. Schofield I. Luxturna Secures English Reimbursement in Record Time. Pink Sheet, Informa, Sept 4, 2019. https://pink.pharmaintelligence.informa.com/PS140779/Luxturna-Secures-English-Reimbursement-In-Record-Time. Accessed September 17, 2020.
3. Holm A, Fernow J. Patient Preferences in Early Drug Development. PREFER; Apr 29, 2019. www.imi-prefer.eu/news/news-item/?tarContentId=782806. Accessed September 17, 2020.
4. McConaghie A. Novartis First to Benefit from NICE Advice on ‘Patient Preference’. PMLive, PMGroup Worldwide Limited. Feb 14, 2019. www.pmlive.com/pharma_news/novartis_first_to_benefit_from_nice_advice_on_patient_preference_1278304. Accessed September 17, 2020.
5. NICE Collaborates With the Canadian Agency for Drugs and Technology in Health to Offer Parallel Scientific Advice. NICE. Feb 6, 2019. www.nice.org.uk/news/article/nice-collaborates-with-the-canadian-agency-for-drugs-and-technology-in-health-to-offer-parallel-scientific-advice. Accessed September 17, 2020.
6. Frequently Asked Questions. NICE. https://www.nice.org.uk/about/what-we-do/life-sciences/scientific-advice/frequently-asked-questions. Accessed September 17, 2020.
7. The Benefit Assessment of Medicinal Products in Accordance with the German Social Code - Federal Joint Committee (G-BA, Gemeinsamer Bundesausschuss). https://www.g-ba.de/english/benefitassessment/ Accessed September 28, 2020.
8. Early Dialogue for a Medicinal Product in Clinical Development. Haute Autorité De Santé. Mar 2016. https://www.has-sante.fr/jcms/c_2623726/en/early-dialogue-ed-for-a-medicinal-product-in-clinical-development. Accessed September 17, 2020.