Combining Real-World and Clinical Trial Data to Study the Effectiveness of Thrombolytics for COVID-19
Trupti Dhumal, MS, BPharm, West Virginia University, Morgantown, WV, USA
The emergence of the COVID-19 pandemic not only compromised an individual’s safety and wellbeing but also led to a global disruption of society’s economy, healthcare services, community engagement, and several other resources. In the context of clinical research, the pandemic conditions presented multiple challenges in the study of investigational drugs due to priority given to immediate patient care. An analysis of COVID-19 clinical trials showed that the majority of trials for COVID-19 therapeutics were not designed to yield actionable information due to low randomization rates and underpowered outcome data. Utilization of real-world data (RWD) in such conditions can help provide supplementary evidence to support clinical studies.
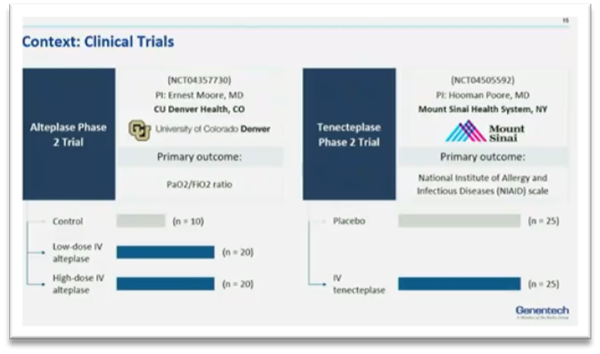
This dynamic workshop moderated by Mitra Corral, MS, MPH (Genentech, USA) and Daniel Sheinson, PhD (Genentech, USA), demonstrated the challenges of conducting randomized controlled trials of study treatments for hospitalized patients with COVID-19 during the pandemic. The workshop also illustrated the application of RWD in addressing the challenges and supplement clinical trial results. The workshop led by 3 presenters Rongrong Wang, Janice Wang, and Marquita Decker-Palmer, introduced a multicenter cohort study and combined datas from a clinical trial of Alteplase (Phase IIa STARS study) along with the RWD acquired from electronic health records of patients treated outside of the trial.
Janice Wang, MD (Hofstra, USA) started with sharing the clinical context related to the cohort study. She further talked about her clinical experiences in conducting trials and challenges faced during COVID-19. “Hospitals were being overwhelmed and makeshift ICUs were created. Patients with acute respiratory distress syndrome were presented at the hospital with different illnesses. Despite supportive care strategies, it became clear that current treatments were not enough,” said Wang. A few of the challenges included staff shortages, obtaining consent, slow recruitment, deployment, thus leading to a significant delay in various clinical study processes.
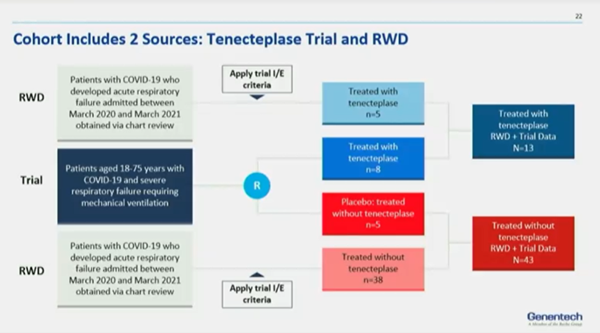
Marquita Decker-Palmer, PhD (Genentech, USA) expanded on the rationale for conducting the cohort study and shed light on the study objectives (Figure 1). She further talked about collaborative efforts involved in clinical operations and other study aspects like design, treatment arm (alteplase and tenecteplase), control arm (placebo), and RWD. The data collection period was 7 months with a multisite collaboration. Researchers built a custom electronic clinical report form to capture RWD based on the clinical trial data elements. These elements were easily extractable from the EHR.
Rongrong Wang, MS (CSL Bewhring, USA) talked about the tenecteplase trial study and gave details on the study participants (n=65, mean age- 63.1). She mentioned that the patients had worse respiratory status. The small sample size posed statistical challenges and thus inverse probability weighting was used to reduce sample issues. Wang moved onto explaining the results and highlighted that supplementing clinical data with RWD was highly efficient and provided greater precision of estimates (Figure 2). Similar techniques were used in the alteplase study along with the same statistical structure. Alteplase, however, was not clinically significant in the combined datasets. The reasons for nonsignificance are attributed to sample size issues and patient severity level. Lastly, she concluded the workshop by stating that RWD can enhance, inform, and accelerate analytic insights from small clinical trials in new therapeutic areas.
Figure 2. Tenecteplace trial and real-world data.
The lessons learned from this study included assessing what research studies to focus next, better understand certain secondary outcomes, better approaching patients with different severity.