Real-World Use of Biosimilar Medicines
Aakash Bipin Gandhi, BPharm, Department of Pharmaceutical Health Services Research, University of Maryland, Baltimore, MD, USA; Vasco Pontinha, MPharm, MA, Department of Pharmacotherapy and Health Outcomes, Virginia Commonwealth University School of Pharmacy, Richmond, VA, USA
Defining the “Bio” in Biosimilars
“Improving population health while maintaining a sustainable healthcare budget is increasingly challenging globally,” Colin Ge, PhD, IQVIA, Plymouth Meeting, PA, USA, said as he kicked off an exciting session that discussed cost savings associated with the use of biosimilars, regulatory challenges associated with their approval, and market opportunities for their adoption across the United States, the European Union, and the Asia Pacific region. [Figure 1]
Figure 1. Session speakers.
As an introduction to biosimilars for the audience, Dr Leah Christl, PhD, Amgen Inc., Washington, DC, USA, highlighted that, “Before learning about what a biosimilar is, it is important to understand what a biosimilar is not.” A biosimilar is not a “biobetter,” “biomimic,” or a generic drug. This means that biosimilars do not aim to establish an improvement in safety and efficacy compared to biologics, they are not molecular copies of an existing biologic, and are evaluated for approval based on stringent regulatory criteria. Biosimilars are “bioequivalent” versions of biologic drugs (ie, drugs that are obtained by means of biotechnology) that show equivalent effectiveness and safety profiles.
“Before learning about what a biosimilar is, it is important to understand what a biosimilar is not.”
— Leah Christl, PhD
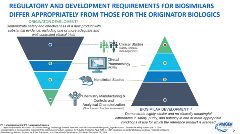
Dr Christl delved further into development and regulatory aspects associated with biosimilars. She noted that, “The similarity in structure and function of biosimilar candidates is established by an iterative process,” which involves continuous tweaking of the manufacturing process until similarity to the reference product is achieved. Other key factors associated with biosimilar development and regulatory approval include safe manufacturing processes, steps to maintain their reliable supply, and post-approval pharmacovigilance. [Figure 2]
Figure 2. Regulatory and development requirements for biosimilars
The Pragmatics of Biologics
The concerns around increasing biologic drug costs warrant discussions for adopting biosimilars. Dr Arnold Vulto, PhD, PharmD, Hospital Pharmacy, The Erasmus University Medical Center, Rotterdam, The Netherlands, explained that biologics are a legal invention that can improve the sustainability of healthcare budgets globally. Savings worth approximately $8-10 billion were generated due to biosimilar uptake in the European Union in 2017.
Figure 3. The 5 "winnings" of biosimilars

Dr Vulto stressed the importance of educating physicians and other healthcare professionals about biosimilars as developmental pathways, for these drugs may differ from those used for generic drugs. To circumvent this problem, the European Union has released detailed guidelines for the use of biosimilars for both clinicians and patients: Region-wide education campaigns were fundamental for increased uptake of biosimilars in the European Union (some countries like Denmark saw adoption rates over 90%). “Overall, the uptake of biosimilars presents certain key wins, including cost benefit, support for a sustainable and competitive market, early patient access to inexpensive biologic treatments, and availability of additional budget for the development of other treatments,” Dr Vulto noted. [Figure 3]
"Overall, the uptake of biosimilars presents certain key wins, including cost benefit, support for a sustainable and competitive market, early patient access to inexpensive biologic treatments, and availability of additional budget for the development of other treatments.”
— Arnold Vulto, PhD, PharmD
Ka-on Lam, MBBS, FRCR, FHKCR, FHKAM, Department of Clinical Oncology, The University of Hong Kong, Hong Kong, stressed that the main factors that prevent a wide adoption of biosimilars in the Asia Pacific region include the absence of regulatory frameworks for their approval and unwritten rules associated with their pricing, market access, and prescribing. In fact, Drs Lam and Vulto agreed that there is no clear consensus or guidance on how biologics may be substituted or switched for biosimilars. Currently, the US Food and Drug Administration, the European Union, and health authorities in the Asia Pacific region have yet to agree on the definitions of switching and interchangeability related to biosimilars.
What’s Next for Biosimilars?
While biosimilars have been available for more than decade, improvements in regulatory pathways for their approval and greater education about their real-word safety and effectiveness are required to foster increased confidence for their adoption globally.
For more information, visit European Medicines Agency video about Biosimilars.

