Bridging the Gap: Pathways for Regulatory and HTA of Histology Independent Therapies
Nazneen Fatima Shaikh, BPharm, DPharm, Department of Pharmaceutical Systems and Policy, West Virginia University, Morgantown, WV, USA
Purva Parab, BPharm, MS, Virginia Commonwealth University, Richmond, VA, USA
A Paradigm Shift in Cancer Treatment
Histology independent therapies, also known as tissue independent or tumor agnostic therapies, target all solid tumors with a certain genomic mutation, regardless of where the primary tumor is in the body. Lotte Steuten, PhD, MSc, Office of Health Economics, London, United Kingdom, started the educational symposium with a brief introduction of the topic, identified challenges, and provided recommendations. Histology independent therapies represent a new era in personalized healthcare and drug development while challenging existing diagnostic and value assessment frameworks. Despite generating paradigm shifts in cancer diagnosis and treatment, these therapies face significant methodological and policy challenges related to evidence development, adoption, value assessment, reimbursement pathways, and immature genomic testing infrastructure.
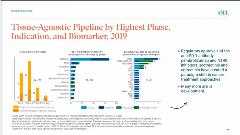
Given the unmet clinical need that exists in patients with rare or ultra-rare diseases, histology independent therapies are able to gain accelerated access through adaptive pathways implemented by regulatory agencies. However, health technology assessment (HTA) bodies pose multiple challenges for evidence requirements to inform coverage and reimbursement decisions. Data on histology independent therapies pipeline in 2019 show a number of products in different stages of the drug lifecycle (Figure 1). With an increasing amount of these therapies coming towards the market, there is a need to find solutions for their unique challenges. Recommendations from Steuten include “better alignment of evidence sources and endpoint requirements, acceptability of post-authorization data collection models, and innovative payment models.” In this educational symposium, speakers provided insight in the current regulatory and HTA landscape for the histology independent therapies from different perspectives.
Figure 1. Histology Independent Therapies Pipeline Products (2019).
Patient Perspective
Bettina Ryll, MD, PhD, Melanoma Patient Network Europe, and Past Chair of the Patient Advocates Working Group, European Society for Medical Oncology, Uppsala, Sweden, began the discussion about heterogeneity in the population and identified the flawed assumption in science where we consider patients as identical to each other or in a controlled environment. Instead heterogeneity should be the starting point and not a finding. She said, “Biology is heterogenous from the beginning; medicine is heterogeneous from the beginning; patients are heterogenous from the beginning; and then you add the therapy on top of it and you’re surprised that we get heterogenous outcomes.”
From a patient’s perspective, histology independent therapy comes closer to the appropriate type of treatment because of the heterogeneity. However, in situations of high unmet need, access can become critical and it helps the patients to know that there are options available so they can make trade-offs. From a patient advocate perspective, these novel therapies challenge the way of evidence generation that highlights the need for adaptation of study design. Pragmatic access models can be considered as one of the options to generate evidence as an intermediate measure and address optimal sequencing of therapies as well. Finally, in order to realize the real benefit of precision medicine, data access, quality control, standard, and re-align incentives needs to be addressed. Ryll concluded, “Form follows function, methodology follows science.”
“Biology is heterogenous from the beginning; medicine is heterogeneous from the beginning; patients are heterogenous from the beginning and then you add the therapy on top of it and you’re surprised that we get heterogenous outcomes.”—Bettina Ryll, MD, PhD
Clinical Perspective
A clinical perspective on the histology independent therapies was provided by Rosa Giuliani, MD, The Clatterbridge Cancer Center, Liverpool, London, England, United Kingdom. Uncertainties with effectiveness of medications in the patient population always exist. One of the reasons could be the extensive list of exclusion criteria of the randomized controlled trials, for instance, patients with comorbidities are generally excluded. However, for a clinician in the real world, uncertainty needs to be addressed on a daily basis. Benefit/risk assessment is generally carried out for decision making based on the available data. Therefore, the shift to precision oncology is critical for the ability to tailor treatment for each patient based on the molecular profile of cancer. “It’s time that we move on for good from the concept that we need to treat groups of patients,” Giuliani said.
To rationally shift to precision oncology, a few key points and their requisites need to be considered (Figure 2). An example was provided of PARPi demonstrating how defining the population based on histology can limit the application of drugs in different types of cancer. Tissue-independent drug development is the new model with agreement from most stakeholders, however, generating solid evidence is needed. Several regulatory agencies and organizations are willing to support innovative medicine.
Figure 2. Rational Shift to Precision Oncology.

Health Economics and the HTA Perspective
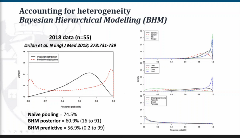
Stephen Palmer, PhD, University of York, York, England, United Kingdom, discussed innovative study designs and analysis methods, relevant examples with challenges, and critical concepts of heterogeneity and uncertainty from the perspective of health economics and HTA. Three innovative study designs used to expedite the process are basket, umbrella, and platform, where basket trials are phase II, single arm and single treatment regimen design. One of the analyses recommended for basket trial was Bayesian hierarchical modelling (BHM). Data from one of the studies of Larotrectinib was presented noting that the efficacy is remarkable however the sample size and variation in types of cancer are concerning. High variation in cancer types in a small group of patients can provide different overall and individual findings.
“It’s time that we move on for good from the concept that we need to treat groups of patients.”—Rosa Giuliani, MD
In Figure 3, the BHM approach is used and the graphs on the right-hand side show differences in response/effect in some types of cancer. For histology independent therapies, HTA would reflect on uncertainty in rare disease by not expecting the same level of evidence as other diseases and balancing the higher uncertainty against lower consequences. With relation to histology independent therapies, alternative approval policies and decision matrix was introduced. In conclusion, Palmer, mentioned opportunities for these novel therapies and emphasized that the studies should be properly designed and analyzed to account for heterogeneity in treatment effects.
Figure 3. Bayesian Hierarchical Modeling.
Payer’s Perspective
The criteria for medical product assessment include therapeutic need, added therapeutic value, and quality of clinical evidence. Entela Xoxi, PhD PharmD MSc, Catholic University of Rome, Roma, Italy, described the payer’s perspective on histology independent therapies and multiple relevant aspects such as measure of innovativeness, progressive authorization lifecycle, managed entry agreement and registry flow, ultimately summarizing the thinking of payer behind all these processes. Based on the criteria for medical product assessment, an innovativeness status is categorized as full, conditional/potential, and noninnovative.
The managed entry agreements are typically of two types: performance-based risk-sharing arrangements; and financial-based arrangements. Performance-based arrangements are more common and meant to manage utilization in the real world and provide evidence regarding decision uncertainty. The thinking of payers related to histology independent therapies is that postmarketing studies or data can be helpful in reducing uncertainty and making informed recommendations. Most payers prefer to manage decisions of uncertainty by negotiating price discounts. Overall, performance-based risk-sharing agreements were considered appropriate for these therapies.
The presentations by speakers were followed by a lively discussion with topics including 1) if histology independent therapies can be considered orphan drugs, 2) coverage with small evidence base and use of performance-based risk-sharing arrangements, 3) histology independent therapies as the last line of treatment and the need to identify its optimum place in the treatment sequence, and 4) application of stratification to study designs. In conclusion, challenges to HTA and their potential solutions were discussed in-depth in an interactive educational session.

