Real-World Evidence of Colonoscopy Screening for Colorectal Cancer Based on a Stepwise Approach
Chisato Hamashima MD, PhD, Teikyo University, Tokyo, Japan, on behalf of the Evidence Review Committee for the Japanese Guidelines for Colorectal Cancer Screening

Background
Colorectal cancer (CRC) poses a significant health burden in developed countries, prompting widespread screening efforts. There are several options for CRC screening, which are as follows: guaiac fecal occult blood test (gFOBT), fecal immunochemical test (FIT), stool DNA test, flexible sigmoidoscopy (FS), colonoscopy, and CT colonography. Although early adoption of new techniques has been expected, CRC mortality reduction should be evaluated before introducing new screening methods into public health programs. However, the evidence obtained from randomized controlled trials (RCTs) is limited to gFOBT and FS, and it requires long-term follow-up. Fecal testing has been used commonly for CRC screening worldwide, and the main method has changed from gFOBT to FIT. The World Endoscopic Organization (WEO) developed a stepwise approach to evaluate a new technique, predicting final results based on test accuracy and program performance in CRC screening.1 This framework was adopted to evaluate FIT, and RCTs have not been evaluated.
"Although early adoption of new techniques has been expected, CRC mortality reduction should be evaluated before introducing new screening methods into public health programs."
What is a stepwise approach?
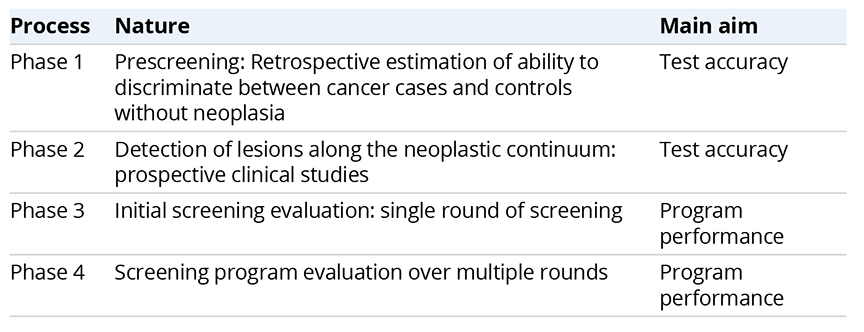
A stepwise approach is an alternative evaluation method when the efficacy/effectiveness of new techniques is not evaluated. Pepe et al originally proposed the basic concept of a stepwise evaluation of cancer screening, which moves from development of a new technique to its adoption in screening programs.2 Based on this concept, the WEO stepwise approach is divided into 4 phases for assessing a new technique for CRC screening (Table 1).1 A comparator should be defined as a standard screening method for evaluating efficacy. In Phases 1 and 2, the new technique’s test accuracy is compared with the standard screening method. For a new technique to be adopted for the screening program, at least equal sensitivity and specificity are required. The program performance results reflect the effectiveness of the new technique, which evaluates mortality reduction by RCTs. In phase 3, program performance is assessed in one round of the screening program and through multiple rounds in phase 4.
Table 1. A stepwise approach for evaluation of CRC screening presented by the World Endoscopic organization1
Methods of a stepwise approach
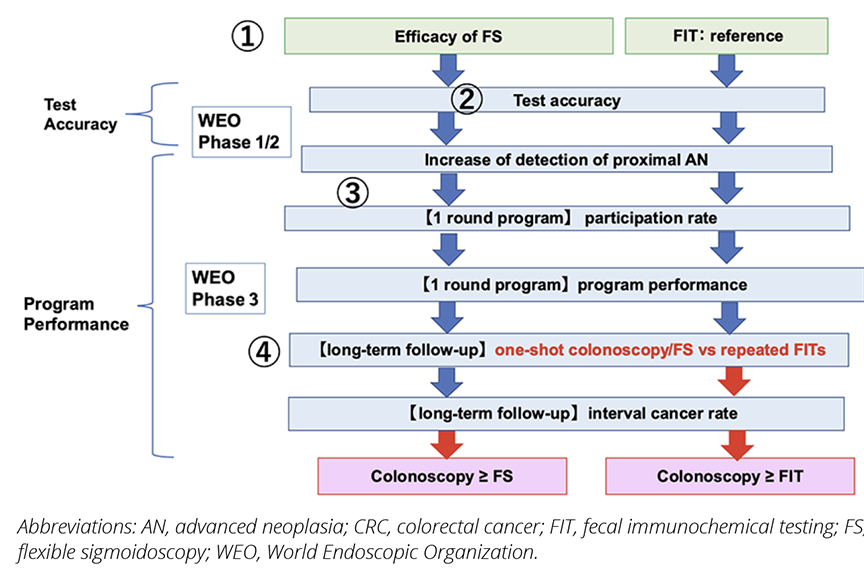
We evaluated the effectiveness of colonoscopy screening following a stepwise approach, modifying the WEO method (Figure 1). In CRC screening, precancerous lesions are the target outcome as well as invasive cancers, which can be removed by colonoscopy before they progress to invasive cancers. The outcomes of these studies are performance indicators, including the participation rate and advanced neoplasia (AN)/CRC detection rates in the program. To assess the effectiveness, we divided the process into the following main steps: Step 1, the efficacy of FS, a comparator, was confirmed for CRC mortality reduction based on RCTs. FIT has also been adopted as another comparator commonly used for CRC screening. In Step 2, test accuracy was compared between colonoscopy and other screening methods. In Step 3, program performance indicators from a single round of screening were compared. Finally, in Step 4, the long-term follow-up results were compared with other methods after one-shot colonoscopy. The screening interval was usually 10 years for colonoscopy, 5 years for FS, and 2 years for FIT. Even in the same observation period, the frequency differed depending on the screening method.
Figure 1. A stepwise approach for evaluation of CRC screening
We defined the inclusion criteria based on population, intervention, comparators, and outcomes (PICO) for selection program performance RCTs. The target of CRC screening is asymptomatic persons. In Step 3, program performance indicators were compared based on single-round RCTs, and a meta-analysis was performed based on intention-to-treat (ITT) and perprotocol (PP) analysis. The result of ITT analysis reflects actual programs that offer population-based screenings.
Evaluation of colonoscopy screening for CRC
Step 1: Based on 15 years of
follow-up, 4 RCTs evaluated the efficacy of FS screening for CRC. The pool
analysis results suggested a 20% mortality reduction from CRC (relative ratio
0.80, 95% CI: 0.72-0.88).3
Step 2: Four studies calculated the
sensitivity of colonoscopy compared with CT colonography (CTC). One study
reported the sensitivity of FIT, FS, CTC, and colonoscopy performed
simultaneously. The sensitivity for AN detection was consistently higher in colonoscopy
than in others, even if the adenoma size was changed.4
Step 3: Candidate articles were
searched for using the MEDLINE, Embase, and Igaku-Cyuo-zasshi (the Japanese
library) from inception to February 2021. From over 7000 articles, those that
did not meet the inclusion criteria, including duplicates and non-RCTs, were
excluded. Finally, 14 RCTs that evaluated the program performance of CRC
screening were selected. All studies evaluated program performance in a
single-round screening. Ten studies from European countries, 2 from the United
States, and 2 from Asian-Pacific countries reported program performance RCTs.
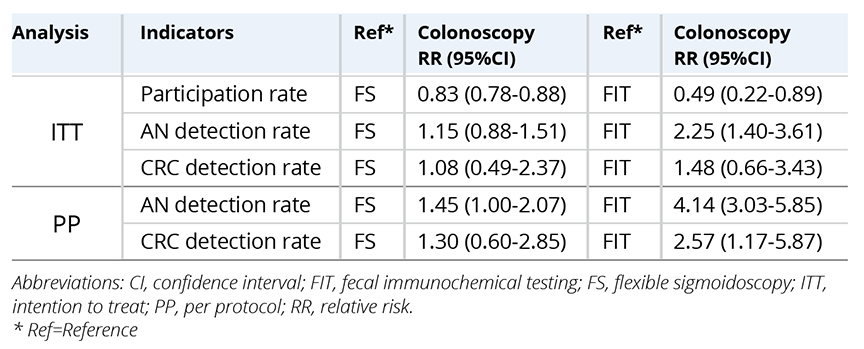
In the ITT analysis, the participation rate of the meta-analysis was lower in
colonoscopy screening than in FIT and FS (Table 2). Although the AN detection rate was higher in colonoscopy
screening than in FIT and FS, the CRC detection rate was higher but not
statistically different. In the PP analysis, AN and CRC detection rates were
higher in colonoscopy than others.
Step 4: Although no RCT existed in multiple-round screening, program performance indicators were compared between one-shot colonoscopy/FS and repeated FITs.5 This study combined the results of 3 randomly selected population-based trials. In the ITT analysis, the AN/CRC detection rate was higher in repeated FIT than in FS and colonoscopy. In the PP analysis, AN detection rates were higher in FS and colonoscopy but CRC detection rates were similar to FIT. On the other hand, the interval cancer rate was lower in colonoscopy than in FS and FIT.
Table 2. Meta-analysis of program performance of colorectal cancer screening
Is it possible to adopt program performance RCTs to evaluate cancer screening?
Even if RCTs have not confirmed efficacy, the program performance can be examined by a head-to-head comparison. When an established method like FS is defined as a comparator, the results of colonoscopy performance could be compared. The success of cancer screening programs depends on the participation rates. The International Agency for Research on Cancer handbook defines participation rates as an essential factor in estimating the effectiveness of cancer screening.6 When colonoscopy is adopted for cancer screening, adherence is lower, leading to a decreased AN/CRC detection rate. Although high sensitivity was confirmed in the colonoscopy screening, the program performance as a cancer screening was deemed insufficient because of the low adherence rates.
"The results of a stepwise approach could predict a screening program’s effectiveness, which is reflected in real-world settings."
Comparison with the Nordic-European Initiative on Colorectal Cancer study
Colonoscopy is anticipated as a new method for CRC screening, with 5 ongoing RCTs yet to yield conclusive results. The Nordic-European Initiative on Colorectal Cancer (NordICC) study, conducted in Poland, Norway, Sweden, and The Netherlands, is one of the RCTs intended to evaluate CRC mortality reduction by colonoscopy screening. They reported intermediate results based on a 10-year follow-up.7 In the ITT analysis, mortality reduction from colorectal cancer could not be observed because of the low adherence in the intervention arm (risk ratio 0.90; 95% CI, 0.64-1.16). Although mortality reduction from CRC was observed in the colonoscopy screening arm at the PP analysis, it was no greater than in those of FS. The meta-analysis results for program performance RCTs are similar to the intermediate results of the NordICC study for colonoscopy screening. Some RCTs reported baseline results and lower participation rates in colonoscopy screening. However, the United States and other countries have observed a difference in participation rates in colonoscopy screening.
Lessons learned
The results of a stepwise approach could predict a screening program’s effectiveness, which is reflected in real-world settings. It is challenging to validate the impact of CRC mortality reduction by colonoscopy screening, and the balance of benefits and harms cannot be determined. The efficacy is still uncertain until the publication of final results of RCTs, which are expected by the late 2020s. Although some countries have already introduced colonoscopy screening, such as the United States and Poland, most countries will be cautious in introducing it as a public health policy.
The Evidence Review Committee for the Japanese Guidelines for Colorectal Cancer Screening
Chisato Hamashima (Chair), Teruhiko Terasawa, Satoyo Hosono, Takafumi Katayama, Koichiro Abe, Keika Hoshi, Toshihiro Tadano, Seiju Sasaki
Funding
This study was supported by the National Cancer Center Research and Development Fund of Japan [grant number A-21, 2023].
References
- Young GP, Senore C, Mandel JS, et al. Recommendations for a step-wise comparative approach to the evaluation of new screening tests for colorectal cancer. Cancer. 2016;12:826-839.
- Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054-1061.
- Juul FE, Cross AJ, Schoen RE, et al. 15-year benefits of sigmoidoscopy screening on colorectal cancer incidence and mortality: a pooled analysis of randomized trials. Ann Intern Med. 2022;175:1525-1533.
- Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241-248.
- Grobbee EJ, van der Vlugt M, van Vuuren AJ, et al. Diagnostic yield of one-time colonoscopy vs one-time flexible sigmoidoscopy vs multiple rounds of mailed fecal immunohistochemical tests in colorectal cancer screening. Clin Gastroenterol Hepatol. 2020;18:667-675.
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Vol. 17. Colorectal cancer screening. Lyon (FR): International Agency for Research on Cancer; 2019.
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387:1547-1556.

