Addressing Health Inequity in Value Assessments: A New Role for HEOR?
Richard Z. Xie, PhD, The Innovation and Value Initiative, Alexandria, VA, USA; Susan dosReis, PhD, University of Maryland School of Pharmacy, Baltimore, MD, USA; Stacey Kowal, MSc, Genentech, Alameda, CA, USA; Louis P. Garrison, PhD, The Comparative Health Outcomes, Policy, and Economics (CHOICE) Institute, University of Washington, Seattle, WA, USA
Introduction
While the specific terminology varies by discipline and country (eg, “health disparities” in the United States, “health inequalities” in the United Kingdom), unjustifiable differences in health, healthcare access and use, and financial protection from healthcare costs among different segments of society have been widely documented across diseases and regions.1-3 The lack of attention to equity considerations has been brought home with the COVID-19 pandemic and calls are increasingly being made to incorporate equity considerations in health-related decision making (eg, in coverage decisions).1,4,5 As a result, the health economics and outcomes research (HEOR) community has recognized our important role in generating key insights, providing data and analytic frameworks that can inform these important decisions.
In May 2021, the Innovation and Value Initiative (IVI) hosted a workshop at the ISPOR Annual Meeting to discuss key considerations and showcase novel methods the HEOR community can leverage to support the consideration of health equity in healthcare decision making. This article summarizes important takeaways from the workshop and provides practical suggestions including (1) ensuring that more representative data inputs are captured, especially from underrepresented communities, (2) considering not only the population-level average impact of a health intervention, but also the equity impacts on different subgroups using tools such as distributional cost-effective analysis, (DCEA), and (3) developing pricing frameworks that can better serve the needs of diverse subpopulations and countries.
Ensuring more representative data inputs are captured
A representative sample of the target patient population is a necessary first step toward understanding health and healthcare disparities. Accounting for patient heterogeneity across people of varying race, ethnicity, and socioeconomic status enables a more comprehensive understanding of patient perspectives, needs, and values. Failure to do so may result in biased findings and healthcare decisions that perpetuate existing systemic disparities.6,7
"The lack of attention to equity considerations has been brought home with the COVID-19 pandemic and calls are increasingly being made to incorporate equity considerations in health-related decision making."
Patient heterogeneity in preferences for healthcare services and interventions is an important focus of the Patient-Driven Values in Healthcare Evaluation (PAVE) Center at the University of Maryland School of Pharmacy. Through a stakeholder-engaged process, PAVE Center researchers incorporate stated preference methods such as discrete choice experiments (DCE) to quantify preference heterogeneity across diverse patient groups.8 Using the PAVE patient-informed value element conceptual model,9 researchers identify value elements (eg, length of treatment, side effects, ability to work) that are prioritized by patients for a given medical condition, and then operationalize these in a quantitative instrument that allows the relative importance of each element to be estimated, stratified by diversity subgroups.10 Subsequently, these insights and findings can be incorporated into methods used to inform health technology assessment, such as economic modeling.
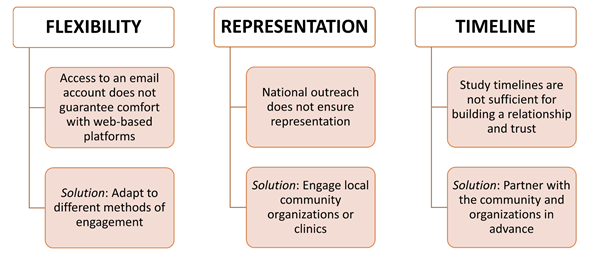
For prospective data collection efforts like the PAVE approach to be successful, ensuring participation from historically underrepresented patient subgroups (eg, those from racial/ethnic minority groups or rural areas) is key. To encourage participation from underrepresented subgroups, researchers should engage with communities of these subgroups and/or healthcare facilities serving these communities before study inception and throughout different study phases. Researchers should make a deliberate effort to understand the barriers (eg, health literacy) to participation from underrepresented patient groups, build trust with community members, and use different engagement and survey methods based on the needs of local communities (Figure 1).
Figure 1. Key Considerations in Overcoming Barriers to Recruiting Representative Patient Samples
Considering population-level impact of a health intervention and the equity impacts on different subgroups
The cost-effectiveness analysis (CEA) is commonly used as a population-level decision tool to inform resource allocation efficiently within a limited budget. However, existing CEA models seek to maximize efficiency by achieving the largest overall gains in health for a given cost and population of interest. However, even if a healthcare intervention is cost-effective at a population level, health and cost outcomes can vary among different population segments depending on various factors, including underlying health risks, uptake, capability to benefit, and, importantly, who will bear the opportunity costs of diverting scarce resources from other uses.6,1
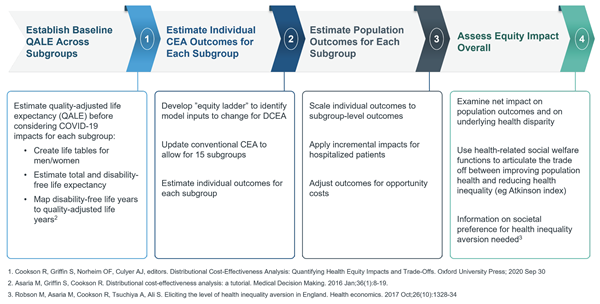
DCEA, an extension of CEA most commonly used outside the United States, allows the comparative valuation of therapeutic alternatives to consider dual objectives, quantifying and comparing tradeoffs between overall gains in health against underlying impacts on health equity.11,12 In an ongoing study, researchers from Genentech demonstrated how the DCEA approach could be implemented in the US setting through a pilot application that examined how the funding of COVID-19 inpatient treatments may impact underlying health disparities.a,13 Figure 2 offers a topline view of the DCEA process implementation based on this specific example. The 4 key steps of the DCEA process are (1) estimating baseline quality-adjusted life expectancy (QALE) across different subgroups, (2) estimating individual CEA outcomes for each subgroup, (3) estimating population outcomes for each subgroup, and (4) assessing the overall equity impact.
Figure 2. Topline View of the DCEA Process
The pilot study illustrated the mechanics and feasibility of applying a DCEA in the United States. Topline results generated important insights for decision making: (1) subgroups with higher levels of social vulnerability had higher quality-adjusted life year (QALY) gains given lower baseline health and higher baseline risk of contracting and dying from COVID-19, and (2) COVID-19 treatments were both cost-effective and had a net positive impact on health equity, given the larger relative gains for more socially vulnerable populations.
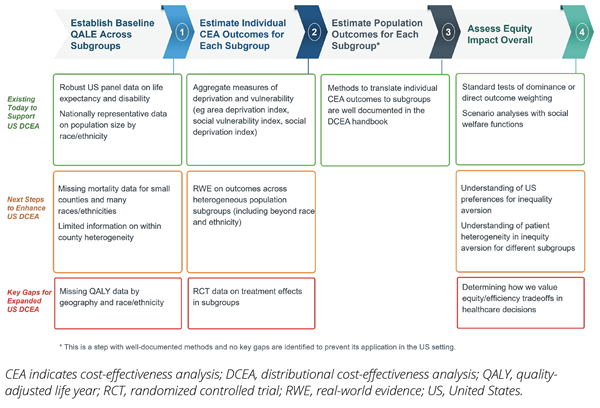
This DCEA application in the US setting demonstrated that the HEOR community in the United States could leverage existing data sources to assess the equity impacts of funding healthcare interventions. However, this work also highlighted key data gaps that need to be filled to further expand use of this approach. For example, in the second step, when researchers modeled intervention impacts on different subgroups, treatment effect data were not readily available by subgroup. So, the research team pivoted and modeled the intervention impacts on different subgroups based on real-world evidence that showed how baseline disease risks and inpatient outcomes were impacted by the level of social vulnerability, across US counties. Figure 3 provides a summary of these data gaps.
Figure 3. US Data Availability and Research Priorities for DCEA
Developing pricing frameworks that serve the needs of diverse subpopulations and countries
While pharmaceuticalb innovations have contributed to improved life expectancy and quality of life, we should be mindful of the equity implications of newly approved therapies.14 Pharmaceutical innovation is potentially rewarding for patients but is often a highly risky venture for public and private investors. In recent years, driven by market incentives and the regulatory environment such as the 21 Century Cures Act, newly approved therapies have increasingly focused on severe rare disease areas with largely unmet needs.15 For example, in 2020, 58% of new drugs approved by the Center for Drug Evaluation and Research of the US Food and Drug Administration were indicated for orphan diseases. Fewer approvals in disease areas that impact broader segments of the population, such as Alzheimer’s disease, are occurring.16,17
In assessing pricing and reimbursement decisions for innovative medicines, value-based pricing frameworks could be adapted to incorporate equity concerns. Economists have long argued for differential pricing in access to pharmaceuticals.18 Income and wealth differences both within the United States and elsewhere influence people’s ability to pay for medicines and thus may mute investment signals to innovators as well as the overall amount available in the research and development ecosystem. Differential pricing, where novel medicines are priced according to the willingness to pay of different subpopulations, can potentially improve access and uptake. Compared with a scenario where a uniform price is set for all subpopulations, differential pricing can potentially reduce disparities within or across countries as well as support equity and dynamic efficiency from a global perspective. In fact, differential pricing is already being applied in the United States, as various insurance payers (eg, Medicare, Medicaid, commercial, and VA) often pay different prices for the same branded drug.19 Differential pricing is, in effect, applying different cost-effectiveness thresholds—which generally affect differences in the ability to pay—for different populations and disease conditions. This redistribution promotes greater access for the less well off, thereby supporting health equity. This is not to say that observed price differences adequately address health disparities, but rather to point out that inequities are recognized, and we need to enhance our analytical and policy tools to better manage them.
To incorporate equity considerations into pricing for novel therapies, decision makers will need to clearly define equity concepts (ie, in terms of outcomes, opportunities, or processes) in specific decision contexts and then develop corresponding measures. With clearly defined equity criteria, improved data inputs and methods such as the DCEA can provide insights into the equity impacts of different alternatives and inform the pricing of novel therapies.
Conclusion
It is imperative that we tackle the widening health disparities in our society.20–22 As members of the HEOR community, we can work with various stakeholders to take immediate action, particularly on improved data inputs and improvement of the methods that inform decision making with potential equity impacts. Promising new methods should continue to be tested and optimized to better address health disparities. In research initiatives, we can: (1) ensure more representative data inputs are captured, especially from underrepresented communities; (2) consider not only the population-level average impact of a health intervention, but the equity impacts on different subgroups using tools such as DCEA; and (3) develop pricing frameworks that can better serve the needs of diverse subpopulations and countries.
References
1. Figueroa JF, Wadhera RK, Lee D, Yeh RW, Sommers BD. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Aff. Published online August 27, 2020:10.1377/hlthaff. doi:10.1377/hlthaff.2020.01040
2. Xie Z, Clair PS, Goldman DP, Joyce G. Racial and ethnic disparities in medication adherence among privately insured patients in the United States. PLoS One. 2019;14(2). doi:10.1371/journal.pone.0212117
3. Patel V, Burns JK, Dhingra M, Tarver L, Kohrt BA, Lund C. Income inequality and depression: a systematic review and meta-analysis of the association and a scoping review of mechanisms. World Psychiatry. 2018;17(1):76-89. doi:10.1002/wps.20492
4. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff. 2020;39(7):1253-1262. doi:10.1377/hlthaff.2020.00598
5. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
6. Ramaekers BLT, Joore MA, Grutters JPC. How should we deal with patient heterogeneity in economic evaluation: a systematic review of national pharmacoeconomic guidelines. Value Health. 2013;16(5):855-862. doi:10.1016/j.jval.2013.02.013
7. Willke RJ, Neumann PJ, Garrison LP, Ramsey SD. Review of recent US value frameworks-a health economics approach: an ISPOR Special Task Force Report [6]. 2018;21(2):155-160. https://doi.org/10.1016/j.jval.2017.12.011
8. Zhou M, Bridges JFP. Explore preference heterogeneity for treatment among people with type 2 diabetes: a comparison of random-parameters and latent-class estimation techniques. J Choice Model. 2019;30:38-49. doi:10.1016/j.jocm.2018.11.002
9. dosReis S, Butler B, Caicedo J, et al. Stakeholder-engaged derivation of patient-informed value elements. Patient. 2020;13(5):611-621. doi:10.1007/s40271-020-00433-8
10. Slejko JF, Hong YD, Sullivan JL, Reed RM, dosReis S. Prioritization and refinement of patient-informed value elements as attributes for chronic obstructive pulmonary disease treatment preferences. Patient. 2021;14(5):569-579. doi:10.1007/s40271-021-00495-2
11. Cookson R, Griffin S, Norheim OF, Culyer AJ. Distributional Cost-Effectiveness Analysis: Quantifying Health Equity Impacts and Trade-Offs. Oxford University Press; 2020. doi:10.1093/MED/9780198838197.001.0001
12. Cookson R, Griffin S, Norheim OF, Culyer AJ, Chalkidou K. Distributional cost-effectiveness analysis comes of age. Value Health. 2021;24(1):118-120. doi:10.1016/J.JVAL.2020.10.001
13. Kowal S, Ng C, Shuldt R, Sheinson D, Cookson R (in press). The Impact of Funding Inpatient Treatment for COVID-19 on Health Equity in the US: A Distributional Cost-Effectiveness Analysis. 2021.
14. Buxbaum JD, Chernew ME, Fendrick AM, Cutler DM. Contributions of public health, pharmaceuticals, and other medical care to US life expectancy changes, 1990-2015. Health Aff. 2020;39(9):1546-1556. doi:10.1377/hlthaff.2020.00284
15. Miller KL, Fermaglich LJ, Maynard J. Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases. Orphanet J Rare Dis. 2021;16(1):1-10. doi:10.1186/S13023-021-01901-6
16. Bountra C, Lee WH, Lezaun J. A New Pharmaceutical Commons: Transforming Drug Discovery.; 2017. Accessed February 28, 2021. https://www.oxfordmartin.ox.ac.uk/downloads/academic/Transforming_Drug_Discovery.pdf
17. Batta A, Kalra BS, Khirasaria R. Trends in FDA drug approvals over last 2 decades: an observational study. J Fam Med Prim Care. 2020;9(1):105. doi:10.4103/JFMPC.JFMPC_578_19
18. Danzon PM, Towse A, Mestre-Ferrandiz J. Value-based differential pricing: efficient prices for drugs in a global context. Health Econ. 2015;24(3):294-301. doi:10.1002/hec.3021
19. A Comparison of Brand-Name Drug Prices Among Selected Federal Programs. Congr Budg Off Rep. Accessed September 3, 2021. www.cbo.gov/publication/56978
20. Mahase E. A decade on from Marmot, why are health inequalities widening? BMJ. 2019;365:l4251. doi:10.1136/BMJ.L4251
21. Ku L, Brantley E. Widening social and health inequalities during the COVID-19 pandemic. JAMA Heal Forum. 2020;1(6):e200721-e200721. doi:10.1001/JAMAHEALTHFORUM.2020.0721
22. Gati SB, Bloomhardt HM, McArthur EA. COVID-19: widening health disparities among pediatric populations. Am J Public Health. 2020;110(9):1358-1359. doi:10.2105/AJPH.2020.305815 00-4

