Impact of Delayed Patient Access to Cancer Treatment
Marco Gross-Langenhoff, PhD, Astellas Pharma, Munich, Germany; Mathias Flume, PhD, MBA, KVWL, Dortmund, Germany; Shilpi Swami, MSc, ConnectHEOR, London, United Kingdom; Jörg Ruof, MD, PhD, MBA, European Access Academy, Basel, Switzerland
Introduction: The Evolving Oncology Space
Cancer remains one of the leading causes of death worldwide, responsible for nearly 10 million deaths in 2020.1 Nearly half of all individuals will be diagnosed with the disease at some point in their lives,2 making continued research and innovation critical. To support and incentivize these efforts, various initiatives and frameworks have been established, for example Cancer Moonshot in the United States, Europe’s Beating Cancer Plan, or the World Health Organization’s Controlling Cancer.2-5 Developments over the last decades led to significant improvements in 5-year survival rates, particularly in regions like the United States.6
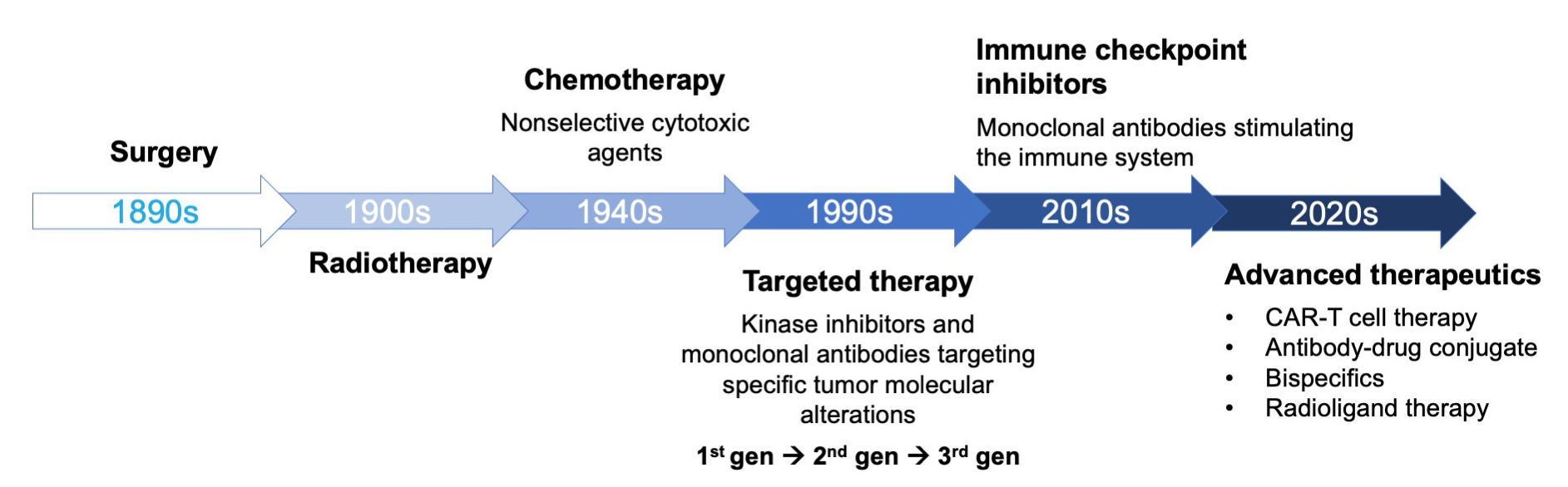
The evolution of cancer treatment has been marked by major breakthroughs over the past century (Figure 1).7 In the early 1900s, surgery and radiotherapy were the primary methods for combating the disease. Since the mid-20th century, chemotherapy was a mainstay of cancer treatment. Scientific advancements in fields such as genomics, transcriptomics, and proteomics enabled a surge in new modalities since the 1990s, such as targeted therapies, be it small molecule kinase inhibitors and monoclonal antibodies. The 2010s introduced immune checkpoint inhibitors, which revolutionized cancer treatment and lay the groundwork for more recent innovations including CAR-T cell therapy, antibody-drug conjugates, bispecific antibodies, and radioligand therapy, all of which have transformed oncology by providing more precise and effective treatment options.7
Despite these advancements, ensuring patient access to new treatments remains a significant challenge. Research activity in oncology is at an all-time high,8 and the number of approved cancer medications by regulatory bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) has profoundly increased since 2000.9,10 However, the availability of these treatments varies across different regions, as will be discussed further, with considerable delays.11,12 Understanding the reasons behind these delays and their implications is crucial for developing solutions for improving patient access to modern oncology treatments. This requires coordinated efforts, as it will be discussed from different perspectives in the following.
Figure 1. The evolution of cancer therapy
A Payer Perspective
To highlight a payer’s perspective, there are multiple challenges in ensuring timely patient access to novel oncology treatments. Recent data comparing access timelines across 6 European countries underscore the significant variability in the time from EMA approval to actual patient access, both between and within countries. This delay persists even after national reimbursement decisions, revealing systemic inefficiencies beyond regulatory approval.13
The payer perspective is not monolithic, but rather spans 3 key dimensions:
- Individual patient perspective: Timely access is critical in oncology, where delays can directly impact survival, especially in advanced disease stages. Despite EMA or national reimbursement, patients often face further delays at the hospital/specialist level.
- Drug portfolio management perspective: Payers must assess whether a new treatment provides added therapeutic value over the current standard of care. The availability of robust clinical data and a sound health technology assessment (HTA) analysis on additional benefits often lags significantly behind regulatory approval. Distinctions between true innovations and “me-too” products are essential for informed decision making and pricing.
- Population and system perspective: Health systems must balance early access with financial sustainability. Granting access at high initial prices can weaken a payer’s negotiating position. Moreover, not all negative reimbursement decisions are budget-driven—some reflect limited evidence on additional benefit versus standard of care.
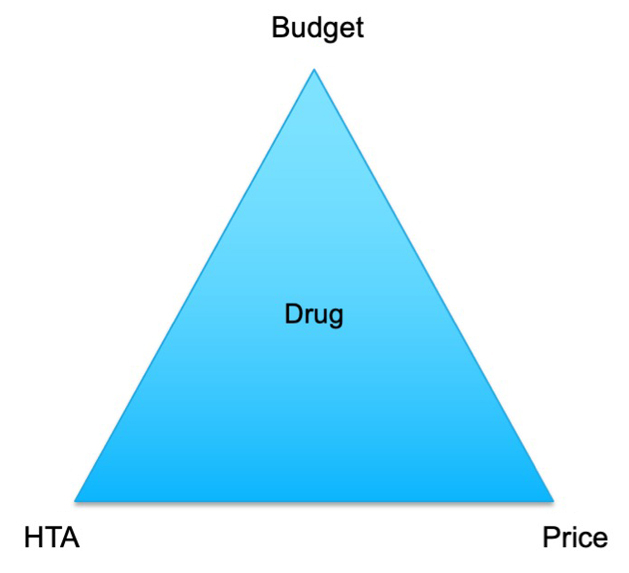
There is no single solution to these access challenges from a payer’s perspective. Payers are committed to delivering effective treatments but must navigate a complex interplay of budget constraints, HTAs, and pricing dynamics (Figure 2).
The upcoming European Joint HTA framework may support more consistent and evidence-driven access decisions, ultimately benefiting both patients and healthcare systems.
Figure 2. The Payer’s Dilemma
A Health Economist Perspective
Delays in access to oncology treatments carry profound health and economic consequences. A health economist’s perspective is critical in identifying, quantifying, and addressing these wider impacts to ensure decisions are not only clinically sound but economically sustainable.
Delays aren’t just regulatory hurdles; they have measurable human and economic consequences, and they present challenges in multiple layers:
- Patient challenges: Heavy out-of-pocket treatment expenditure, indirect costs, and outcomes such as early progression, shorter survival, and low quality of life
- Societal impact: Caregiver burden, productivity loss, and equity considerations
- Payer dilemma: Budget constraints, HTA processes, population health maximization, and pricing negotiations
- Industry perspective: Pricing and reimbursement strategies, financial uncertainty, and slowdown in investment in research and development
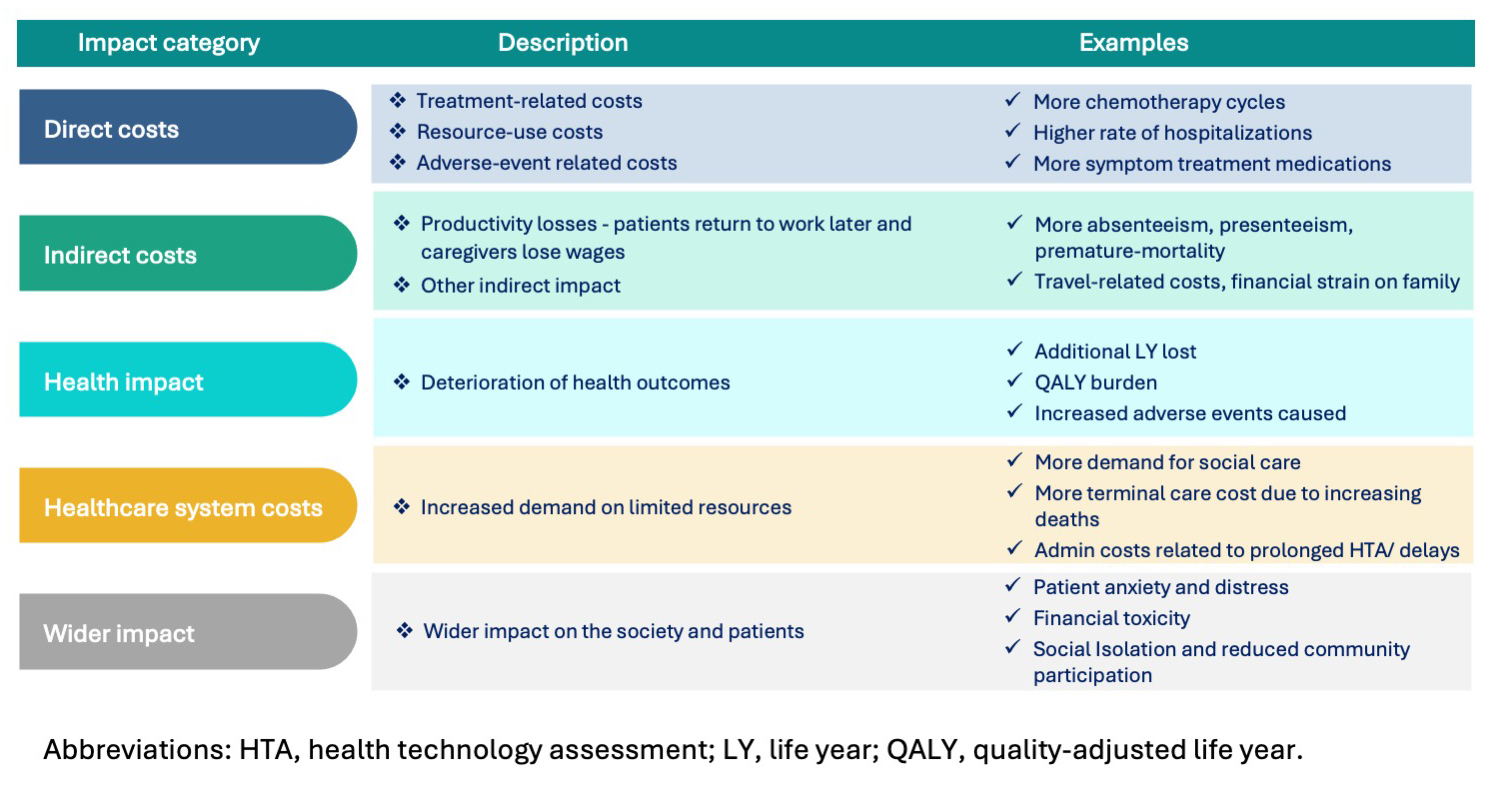
The health economist considers multiple perspectives, ranging from clinical to economic to policy-driven, and synthesizes them into actionable insights for decision making. The key impact categories to measure the full impact of delays include direct costs, indirect costs, health outcomes, healthcare system costs, and wider impact on society (Figure 3).
Figure 3. Representation of categories when measuring the impact of delays in access to treatment in oncology
Although there are a limited number of studies, the existing evidence confirms these modeled assumptions with global real-world data14:
- Globally, an incremental societal value ranging from $38,000 to more than $1 million per newly treated patient per month, due to early reimbursement.15
- For each year of drug approval acceleration, a median of 79,920 life-years per drug could be saved worldwide.16
- The societal value of life-days lost per patient ranged from $32,148 in Italy to $101,565 in Australia.17
- Across various countries, delayed access to oncology drugs potentially resulted in the loss of more than 30,000 life years. Total potential progression-free life years lost were 48,037 in Canada. The worst delays (~15 years) resulted in 5.76 lost life years per patient and 4.14 lost quality-adjusted life years (QALYs) per patient in China.18-20
- In Canada, delays affected 6400 patients, who lost up to 1740 life years and 1122 QALYs (valued at CA$112 million). Productivity loss was estimated at CA$106 million.21
Delays have cost healthcare systems millions in lost productivity and economic burden. Patients suffer the most due to higher mortality, financial strain, and reduced quality of life. However, access alone isn’t enough. We need to ensure successful implementation and continuously evaluate real-world impact.
An Academia Perspective
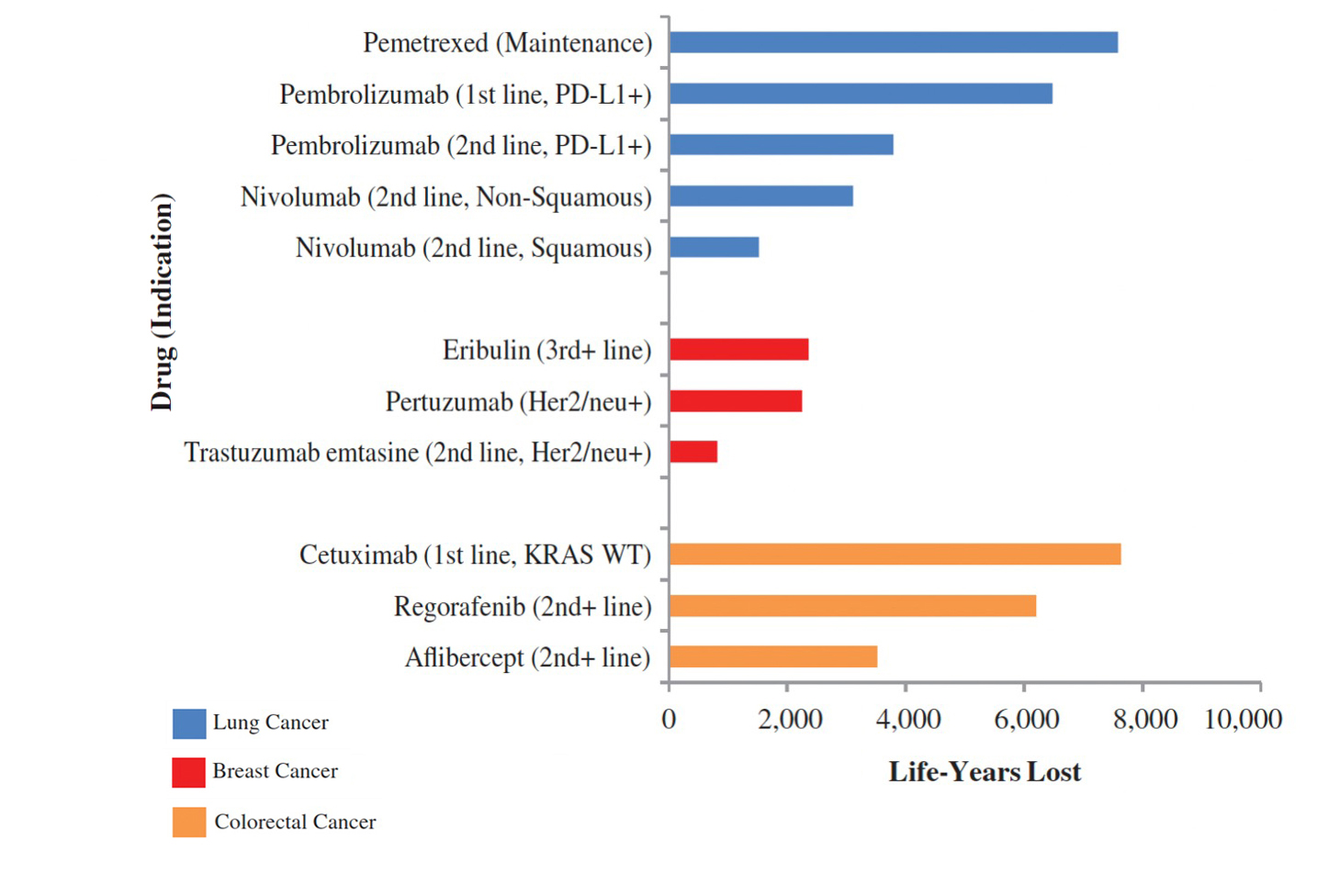
Further data show considerable differences in the time to access and implications for patients: An analysis of 167 EMA-approved drugs (2019-2022) revealed a European Union (EU) average of 474 days from EMA approval to market access, with significant variations between countries—Germany having the shortest access time (47) and Poland (770) among the longest.22 Analysis from Canada showed prolonged time from proof of efficacy to first public funding, resulting in considerable years of life lost (Figure 4).19
Figure 4. Delay from proof of efficacy to health technology assessment process to first public funding19
A variety of options are available to improve the access of patients to innovative medicines:
- Optimizing early access schemes: France reshaped its Early Access schemes in 2021 with 3 routes of Compassionate Use Authorization (CUA), Early Access Authorization (EAA), and Direct Access (DA) with different timings in relation to marketing authorization. In addition to the benefit of having early access to treatment, an analysis showed that majority of medicines included into the early access scheme turned into Service Médical Rendu (SMR) important assessment later.23,24
- Accelerating regulatory timelines: Project Orbits is an initiative with global regulatory reach through which countries can benefit from the usually earlier submission to the US Food and Drug Administration (FDA). It was launched by the FDA’s Oncology Center of Excellence in 2019, and it seeks to expedite patient access by coordinating regulatory reviews across multiple countries, including the United States, United Kingdom, Australia, Brazil, Canada, Israel, Singapore, and Switzerland. As an example, this approach has significantly reduced regulatory timelines in Switzerland, reducing the submission gap from 168 to 33 days and median review times from 314 to 235 days.25
- Alignment of HTA requirements: In Europe, an overhaul of HTAs through the introduction of the Joint Clinical Assessment (JCA) through the EU HTA Regulation aims to standardize evaluations across member states and therefore accelerate access to medicines. However, this might be possible only in the mid- to long-term, whereas in the short-term it might increase the burden and delay processes. The experience was similar at the time of the introduction of the EMA.26
- Deletion of the fourth hurdle: A final example of how to potentially speed up access to cancer medicines is by revisiting the so-called fourth hurdle, which refers to having access to medicines only after completion of the HTA. In Germany, access is immediately granted at the start of the respective HTA rather than after its completion, leading to the quick access to medicines in Germany as shown in some of the above-mentioned analyses.22
Conclusions
Delays in oncology treatment access can have measurable consequences: impacting survival, quality of life, and healthcare system efficiency. While progress has been made, disparities remain. A multistakeholder approach is essential to accelerate access, supported by early evidence generation, aligned HTA and pricing processes, and adaptive policy reforms. Timely access isn’t just a regulatory goal—it’s a necessity for delivering the full value of innovation to patients, health systems, and society.
Ultimately, the benefits of therapeutic improvements in oncology, often with life-saving potential, should be available for patients without delay.
The ISPOR Oncology Special Interest Group (SIG) was established to identify new trends and methodological challenges in oncology HEOR with the intent of supporting education, awareness, and community engagement while working towards the development of recommendations to address them. The SIG aims to advance clinical and methodological knowledge for proper clinical and economic evaluation of oncology treatments and diagnostic tools. This article reflects the key ideas presented by the authors during the group’s recent webinar on delayed access to cancer treatments.
References
- Cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancer. Published February 3, 2025. Accessed April 1, 2025.
- Cancer risk statistics. Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/risk. Accessed April 1, 2025.
- Cancer moonshot. National Cancer Institute. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative. Accessed April 1, 2025.
- Europe’s Beating Cancer Plan: Communication from the commission to the European Parliament and the Council. https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf. Accessed April 1, 2025.
- Controlling cancer. World Health Organization. https://www.who.int/activities/controlling-cancer. Accessed April 1, 2025.
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9: 1300. doi: 10.3389/fphar.2018.01300. PMID: 30483135; PMCID: PMC6243123.
- Global oncology trends 2024: outlook to 2028. IQVIA. https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/global-oncology-trends-2024. Published May 28, 2024. Accessed April 1, 2025.
- Scott EC, Baines AC, Gong Y, et al.. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat Rev Drug Discov. 2023;22(8):625-640. doi: 10.1038/s41573-023-00723-4.
- Addressing challenges in access to oncology medicines: analytical report. OECD. https://www.oecd.org/content/dam/oecd/en/publications/reports/2020/04/addressing-challenges-in-access-to-oncology-medicines_5f0e2f62/699520d0-en.pdf. Published 2020. Accessed April 1, 2025.
- Zhang Y, Hueser HC, Hernandez I. Comparing the approval and coverage decisions of new oncology drugs in the United States and other selected countries. J Manag Care Spec Pharm. 2017;23(2):247-254. doi: 10.18553/jmcp.2017.23.2.247
- Newton M, Stoddart K, Travaglio M, Troein P. EFPIA patients W.A.I.T indicator 2023 survey. IQVIA. https://efpia.eu/media/vtapbere/efpia-patient-wait-indicator-2024.pdf. Published June 2024. Accessed April 1, 2025.
- Vancoppenolle JM, Franzen N, Koole SN, Retèl VP, van Harten WH. Differences in time to patient access to innovative cancer medicines in six European countries. Int J Cancer. 2024;154(5):886-894. doi: 10.1002/ijc.34753
- Swami S, Lakhsmi R, Sharma R, Mohseninejad L. HTA146 How delays in access are losing the battle against cancer: the impact on patient and economic outcomes. Value Health. 2024;27(12):S382.
- Lakdawalla DN, Chou JW, Linthicum MT, MacEwan JP, Zhang J, Goldman DP. Evaluating expected costs and benefits of granting access to new treatments on the basis of progression-free survival in non-small-cell lung cancer. JAMA Oncol. 2015;1(2):196-202. doi: 10.1001/jamaoncol.2015.0203
- Stewart DJ, Stewart AA, Wheatley-Price P, et al. The importance of greater speed in drug development for advanced malignancies. Cancer Med. 2018;7(5):1824-1836. doi: 10.1002/cam4.1454
- Leinwand B, Sollano J, Doherty, JP, et al. Pcn402 the clinical and economic consequences of delays in reimbursement for select novel cancer therapeutics in Canada, Italy, and Australia. Value Health. 2019;22:S514. 10.1016/j.Jval.2019.09.597
- Uyl-de Groot CA, Heine R, Krol M, Verweij J. Unequal access to newly registered cancer drugs leads to potential loss of life-years in Europe. Cancers (Basel). 2020;12(8):2313. doi: 10.3390/cancers12082313
- Gotfrit J, Shin JJW, Mallick R, Stewart DJ, Wheatley-Price P. Potential life-years lost: the impact of the cancer drug regulatory and funding process in Canada. Oncologist. 2020;25(1):e130-e137. doi: 10.1634/theoncologist.2019-0314
- Zhu X, Liu B. Launch delay of new drugs in China and effect on patients’ health. Clin Ther. 2020;42(9):1750-1761.e7. doi: 10.1016/j.clinthera.2020.06.023
- Vanderpuye-Orgle J, Erim D, Qian Y, et al. Estimating the impact of delayed access to oncology drugs on patient outcomes in Canada. Oncol Ther. 2022;10(1):195-210. doi: 10.1007/s40487-022-00187-3
- Hecken J. Blinde Flecken im AMNOT-Verfahren. Gemeinsamer Bundesausschuss. https://caas.content.dak.de/caas/v1/media/76616/data/663635bc26bf2817287dd22430f7cf37/vortrag-amnog-report-2024-hecken.pdf. Published July 19, 2024. Accessed April 1, 2025.
- Autorisation d’accès précoce, autorisation d’accès compassionnel et cadre de prescription compassionnelle. Ministère du travail, de la Santé, des Solidarités et des Familles. https://sante.gouv.fr/soins-et-maladies/medicaments/professionnels-de-sante/autorisation-de-mise-sur-le-marche/article/autorisation-d-acces-precoce-autorisation-d-acces-compassionnel-et-cadre-de. Published April 24, 2025. Accessed April 1, 2025.
- Abdelghani I, Jdidi H, Chachoua L, et al. HTA353 Analysis of the impact of early access decisions on pricing and reimbursement decisions in France. Value Health. 2023;26(12):S388.
- Zosso-Pavic M, Li Q, Atiek E, Wolfer A, Rohr UP. Effect of Project Orbis participation by the Swiss regulator on submission gaps, review times, and drug approval decisions between 2020 and 2022: a comparative analysis. Lancet Oncol. 2024;25(6):770-778. doi: 10.1016/S1470-2045(24)00158-X
- Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU. Official Journal of the European Union. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R2282. Published December 22, 2021. Accessed April 1, 2025.

