Solving the Real-World Evidence Challenge for US Payers: A Call to Action for Pharma
Charles Makin, BSPharm, MS, MBA, MM, Boehringer Ingelheim, CT, USA; Brian Sweet, BS Pharm, MBS, Bristol Myers Squibb, Naples, FL, USA; Mats Rosenlund, PhD, MPH, Daiichi Sankyo, Stockholm, Sweden; Matteo Cefaliello, BSc, MSc, IQVIA, London, UK; Daniel Newsome, BSc, IQVIA, London, UK; Nuno Apóstolo, PhD, MSc, BSc, IQVIA, Coimbra, Portugal; Nicole Rickett, PhD, MSc, BSc, IQVIA, London, UK
The views in this text represent the opinions of the authors, and do not represent the views or opinions of their respective organizations.
Introduction
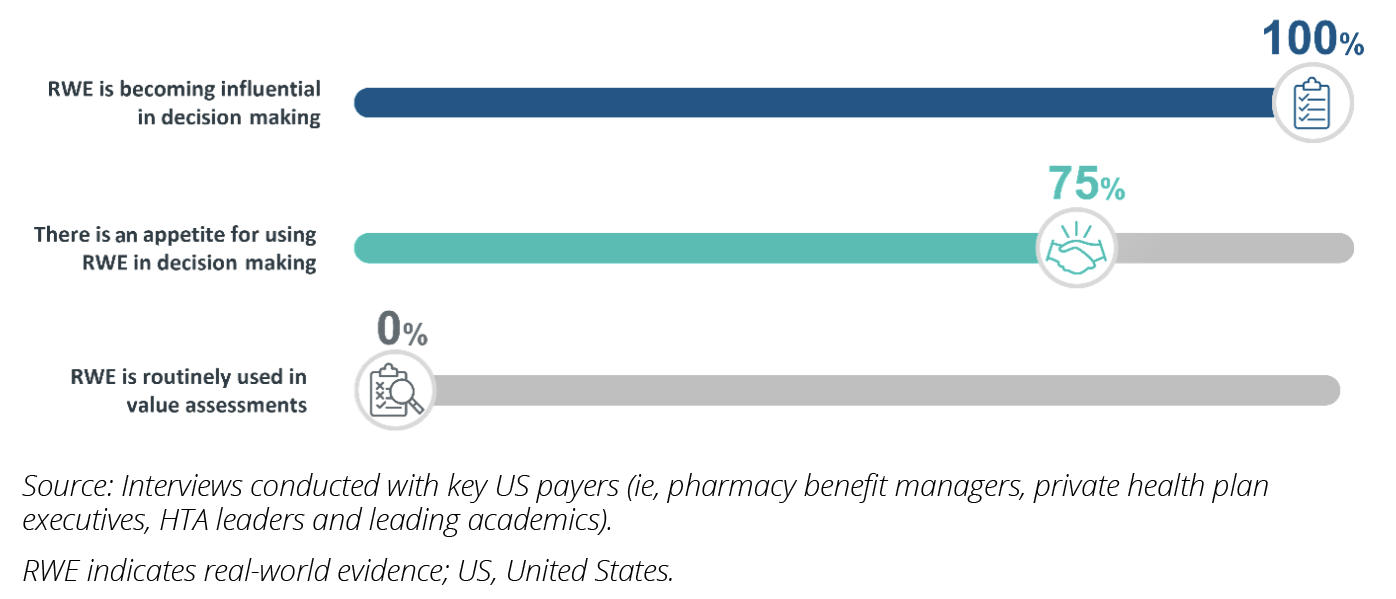
The healthcare landscape continues to grapple with marketplace economic pressures to control spending, magnified by the continued financial impacts of the COVID-19 pandemic. In addition, with the impending implementation of the Inflation Reduction Act, payers are under increasing strain to deliver healthcare in a more cost-effective manner. In this changing landscape, real-world evidence (RWE) presents payers with a powerful tool, which, when combined with randomized clinical trial data, can help better interpret the value of therapies for reimbursement decision making. However, RWE utilization in payer value assessments remains low, despite its potential (Figure 1).
This pharmaceutical industry perspective, authored by RWE experts from industry and IQVIA, part of the “RWE Leadership Forum,” aims to identify and address barriers hindering the widespread adoption of RWE in healthcare decision making. Through a collection of informal interviews with key US healthcare financing stakeholders (pharmacy benefit managers [PBMs], private health plan executives, health technology assessment [HTA] leaders, and leading academics) and a literature review supplemented by author experience, this paper examines the current and future state of RWE, exploring challenges, opportunities, and recommendations to enhance its uptake.
Figure 1: Common sentiments on the evolving use of RWE among US payers.
The evolving role of RWE
Increasing marketplace pressures to reduce cost and further demonstrate value are driving the need for additional evidence generation. In August 2022, the expansive Inflation Reduction Act was signed into law, including healthcare-specific provisions aimed at reducing drug costs for the federal government and patients.1 These include granting the Centers for Medicare & Medicaid Services (CMS) powers to negotiate drug pricing, a Medicare Part D redesign, maximum patient out-of-pocket caps, and prescription drug inflation rebates. These provisions and subsequent increased pricing pressures will be key drivers altering current evidence requirements and evaluation standards.
With healthcare spending in focus globally, payers are seeking innovative solutions for evidence appraisal, cost modeling, and contracting. Drug price negotiation in particular is expected to be a catalyst for enhanced rigor in evidence generation. With a significantly higher proportion of the cost of drugs falling on commercial payers, more comprehensive bodies of evidence and value demonstration will be required to inform decisions and justify risks.
High-quality RWE presents an opportunity to transform healthcare decision making and unlock improved population health management. Recent technological advancements are now enabling faster, more patient-centric and cost-effective generation of observational evidence, as newer and more effective modalities of data linkage combined with improved real-world data (RWD) collection through wearables and electronic medical record systems are increasing data availability. This is leading several organizations to create large in-house real-world datasets covering their target populations and enabling evidence-based healthcare decision making.
RWE has gained significant traction within regulatory submissions in the past decade, with the US Food and Drug Administration (FDA) releasing guidelines on its use in submissions and 90% of new drug approvals in the United States including RWE in 2020.2 However, the incorporation of RWE in decision making by US payers has been slow and limited to select cases, which is further illustrated by the summary of our US payer interviews as outlined below.
Challenges and barriers
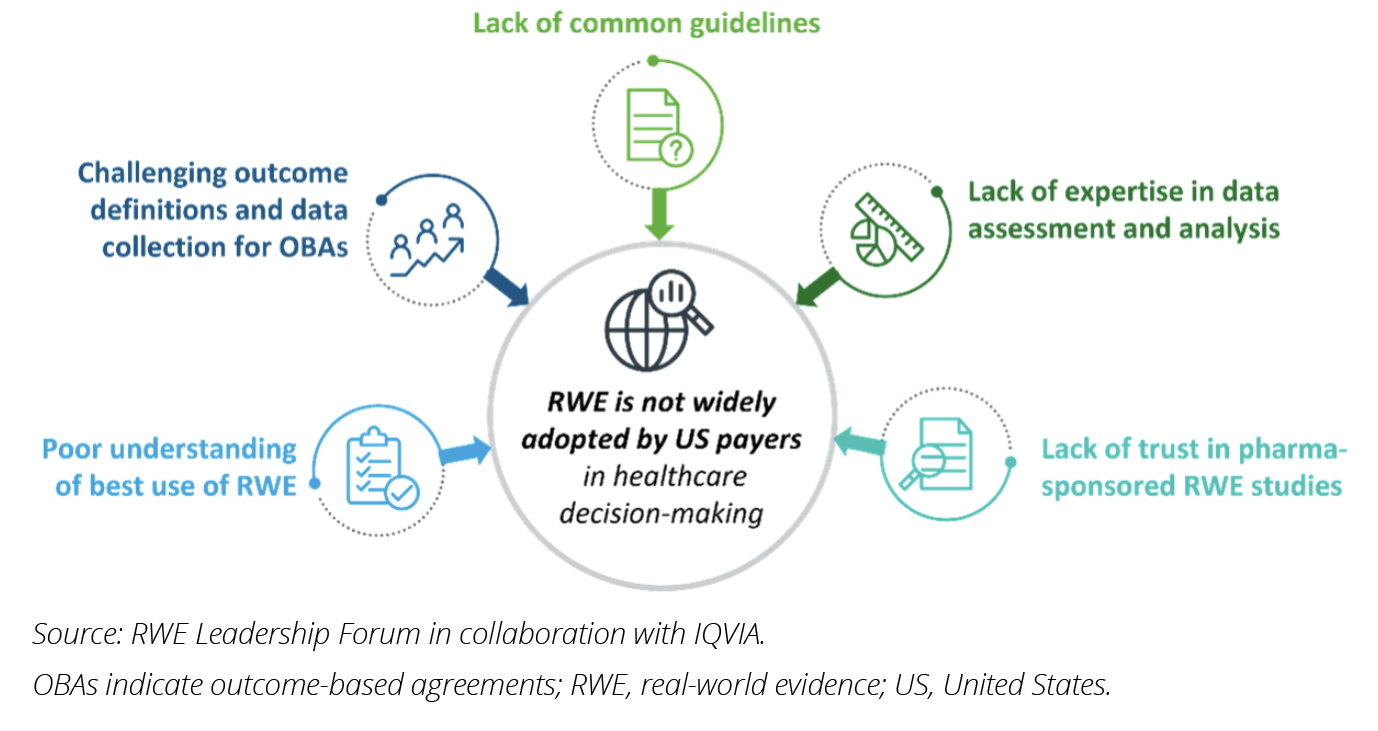
Five key challenges currently hindering the routine use of RWE were identified through expert interviews with US payers (Figure 2). Understanding and addressing these challenges will be the first step in paving the way for RWE to be better utilized in future payer value assessments, thus unlocking its full potential for substantive applications in healthcare decision making.
1. There is a poor understanding of optimal use of RWE for payer decisions. The true value of high-quality RWE can only be realized when applied to the appropriate situations to support decision making. Due to limited opportunities to collect relevant data before market authorization, the use of RWE in initial submissions beyond natural history and standard-of-care data is limited. The highest value for RWE is likely during reassessments of drugs, in situations such as formulary evaluations and treatment sequencing decisions, as there are far greater opportunities to collect relevant RWD at this stage.3 Some stakeholders, such as the Institute for Clinical and Economic Review (ICER), have recognized the value of RWE in reassessments and moving towards a life-cycle approach with regular reassessments.4 However, the majority of respondents stated that they do not regularly use RWE in drug value assessments and pointed to poor communication by pharma companies on the specific value of RWE for payers.
2. Outcomes-based agreements’ real-world outcome metrics are challenging to define and measure. Innovative contracting, particularly through outcomes-based agreements, has emerged in recent years as a potential solution to address the increased financial pressure and uncertainty over long-term efficacy presented by novel precision medicines. RWE serves as the basis for these agreements, providing information on the real-world performance of therapies. All interviewed payers expressed high interest in defining outcomes-based agreements centered around RWE. However, the adoption of such agreements has been slow due to difficulties in defining and measuring relevant real-world outcome metrics. The lengthy timelines for determining the success and value of therapies in these agreements also pose challenges to decision makers who face shorter-term budget review cycles. As a result, simpler rebate models are often preferred over high-risk outcomes-based agreements.
3. There is a lack of common guidelines for evaluating RWE during value assessments. While some frameworks for real-world study execution exist,5,6 there are currently no consensus guidelines for US payers on the interpretation and evaluation of RWE. Varying guidelines originally designed to address regulatory requirements or custom-made value assessment frameworks are typically used among payers, and many are using tools designed for evaluating randomized clinical trials, to assess RWE, despite significant methodological and structural differences between the study types. Additionally, there are currently no tools available for interpreting RWE in combination with evidence from randomized clinical trials.7
4. Payers lack expertise in data assessment and analysis for RWE studies. Even within the regulatory setting, there are widespread and varied concerns over the methodological approaches used in real-world studies, including data collection issues, study design flaws, and poor analytical approaches.3,7 Furthermore, several respondents noted a lack of consideration for payer requirements, such as poor representativeness or relevance to payers’ populations of interest.
5. There is a historic lack of trust in pharma-sponsored RWE. Poor transparency in the processes by which pharmaceutical companies generate and publish real-world studies has contributed to this lack of trust and was consistently cited as a key reason for not including RWE in value assessments. The absence of regulatory requirements to publish intentions and protocols prior to conducting RWE studies, as is required for randomized clinical trials, raises concerns over selective publishing and potential conflicts of interest.3,7,8 Indeed, some payers highlighted they would only accept RWE in a value assessment if the research had no affiliation with pharma and was published in a reputable peer-reviewed journal.
Figure 2: Five key challenges hindering widespread adoption of RWE use by US payers.
Recommendations to pharmaceutical companies
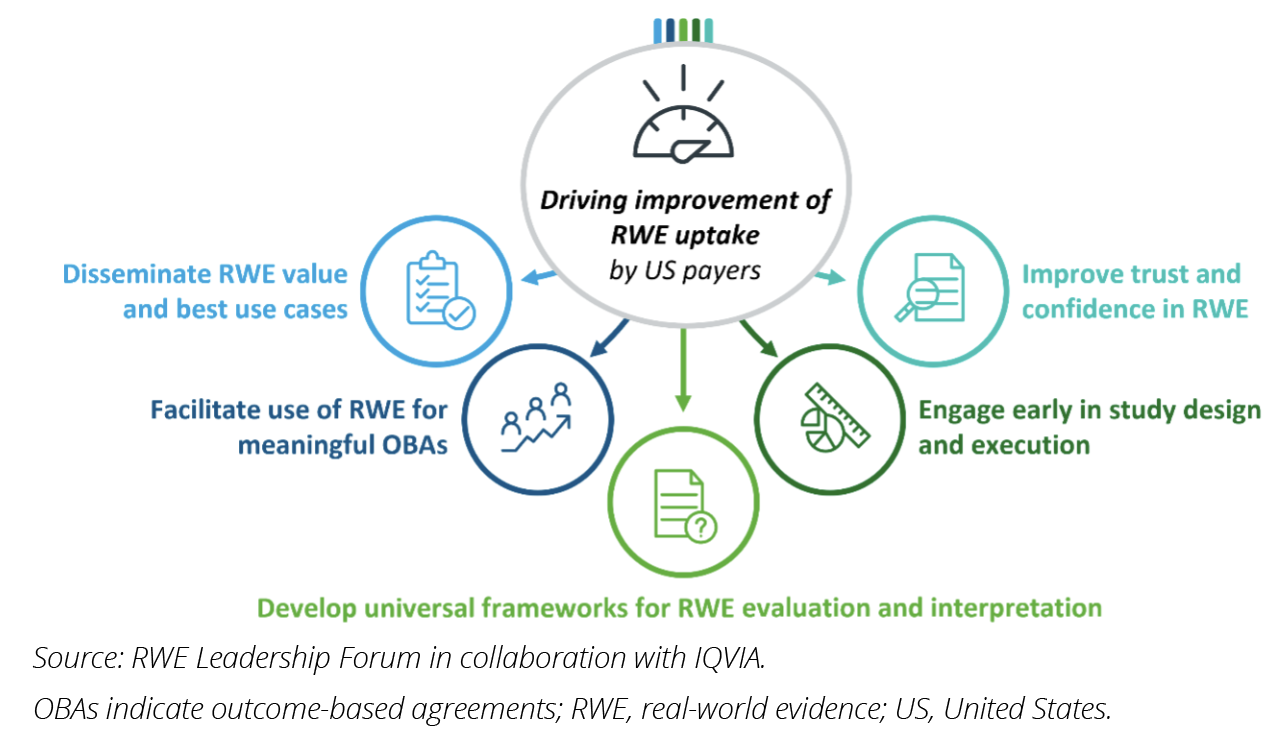
The pharmaceutical industry will need to actively address these barriers to improve value assessment of new medicines. We recommend 5 specific actions to improve RWE utilization and realize its potential in decision making (Figure 3):
1. Engage early: Collaborate with payers in study design and execution to foster a productive relationship. By involving payers in data collection and/or analysis, pharma can reduce payer hesitation and educate on the best practices for data generation and usage.
2. Enhance trust and confidence in RWE: Improve transparency and scientific merit of RWE. Where possible, make study details publicly available, including intent, data sources, analytical strategy, and expected output. Engage payers throughout the study, address their requirements, and be open to critique on methodological approaches.
3. Invest in payer education and universal frameworks: Educate payers on evaluating and interpreting RWE. Provide tools, education, and expertise to enhance payer capabilities. Support the development of consensus guidelines and frameworks for evaluating RWE in standalone studies or combined with randomized clinical trial data.
4. Discuss the value of RWE for payers: Translate RWE findings into payer-relevant messaging, emphasizing cost-effectiveness and impact on specific patient cohorts. Educate payers on the value of RWE throughout a product’s life cycle, including real-world performance, safety monitoring, and drug interactions.
5. Facilitate meaningful outcome-based agreements: Address the lack of appropriate outcome metrics, measurement systems, and data availability for outcomes-based agreements. Engage with payers during study design to ensure outcomes and measurements are relevant and meaningful for patients and payers. Foster collaboration among key stakeholders, including healthcare providers and patient advocacy groups, to define data collection structures and invest in universal optimized data infrastructure (eg, Observational Medical Outcomes Partnership).
Figure 3: Five recommendations for pharmaceutical companies to directly address the 5 challenges hindering RWE uptake in critical appraisals.
Conclusion
As interest in RWE continues to grow and data collection and analysis capabilities become more powerful, RWE offers immense potential for payers to better understand the true performance of therapies. Economic pressures and the need for long-term drug evaluations will increase the importance of considering RWE in value assessments. However, barriers such as payer mistrust, inadequate guidelines and education, and difficulty incorporating RWE into supply contracts have hindered it from reaching its potential in the US healthcare system. By building this trust, investing in education of its applicability, and opening dialogues with payers to guide mutually beneficial studies, efforts from pharma will facilitate better adoption of RWE in drug value assessments and ultimately improve patient outcomes by allowing decision makers to make data-driven treatment decisions and select the best therapy for every patient based on the full body of evidence.
References
1. H.R.5376 - Inflation Reduction Act of 2022. 117th Congress (2021-2022). Accessed January 3, 2024. https://www.congress.gov/bill/117th-congress/house-bill/5376/text
2. Purpura CA, Garry EM, Honig N, Case A, Rassen JA. The role of real-world evidence in FDA-approved new drug and biologics license applications. Clin Pharmacol Ther. 2021;111(1):135-144.
3. Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE Special Task Force on Real-World Evidence in Health Care Decision Making. Value Health. 2017;20(8):1003-1008.
4. Jaksa A, Boudek L, Carlson J, et al. Key learnings from Institute for Clinical and Economic Review’s real-world evidence reassessment pilot. Int J Technol Assess Health Care. 2022;38(1):e32.
5. National Institute for Health and Care Excellence. NICE Real-World Evidence Framework. Corporate document [ECD9]. Published June 23, 2022. Accessed January 3, 2024. https://www.nice.org.uk/corporate/ecd9/chapter/overview
6. Sweet B, Tadlock CG, Waugh W, Hess A, Nguyen A. The WellPoint Outcomes Based FormularySM: enhancing the health technology assessment process. J Med Econ. 2005; 8(1-4):13-25.
7. Roberts M, Ferguson G. Real-world evidence: bridging gaps in evidence to guide payer decisions. Pharmacoecon Open. 2021;5(1):3-11.
8. Malone DC, Brown M, Hurwitz JT, Peters L, Graff JS. Real-world evidence: useful in the real world of US payer decision making? How? when? and what studies? Value Health. 2018;21(3):326-333.

