Examining the Cost-Effectiveness in Oncology Studies
Trupti Dhumal, MS, BPharm, West Virginia University, Morgantown, WV, USA
With the advancement in medical oncology and introduction of novel therapeutics, there is an ongoing debate between pricing and determining the value of a therapeutic. A lot of emphasis is given on generating evidence for cancer treatments to facilitate appropriate medical decision making. National agencies are continuously assessing approaches for reducing excessive cost in cancer treatment and determining its value to the public.
Koen Degeling, PhD (Lumen Value & Access, The Netherlands) kicked off this exciting session by introducing the speakers and facilitating an important discussion regarding the recently conducted cost-effectiveness analyses in oncology. The purpose was to bring together 4 different cost studies focused on a general theme of reducing excessive treatment cost and exploring treatment alternatives.
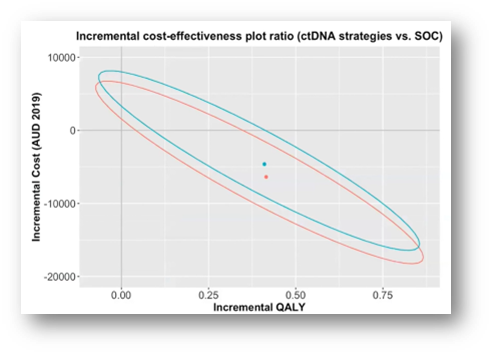
Medical oncologist Yat Hang To (Walter and Eliza Hall Institute of Medical Research, Australia) raised an important issue of overtreatment in stage 2 colon cancer stating that too many patients are receiving adjuvant chemotherapy without actually benefiting from it. He presented an innovative biomarker ctDNA approach to guide the adjuvant chemotherapy decision making by only treating patients who are ctDNA positive. The focus was to generate evidence on the cost-effectiveness of ctDNA compared to the standard of care (SOC) by conducting a cost-utility analysis using a cohort of 65-year old Australian patients from a healthcare perspective. Compared to the SOC, the ctDNA and preference-sensitive ctDNA strategies increased quality-adjusted life years (QALYs) by 0.20 and dominated the SOC, showing a great potential to be cost effective, thus reducing the excessive treatment cost (Figure 1). To remarked that, “In stage 2 colon cancer of the one third of patients receiving chemotherapy, only a small proportion of patients is actually benefiting in terms of reduced risk of reduction; meaning a vast majority of patients exposed to unnecessary treatment result in health and financial toxicities.”
Figure 1. Results of probabilistic analysis
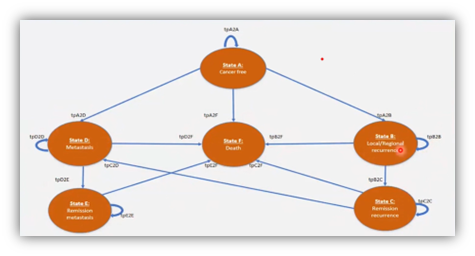
Currently, a majority of the cost studies in oncology focus on chemotherapeutic treatments rather than surgical alternatives. Phu Duy Pham, MPH (German Cancer Research Center, Germany) provided insightful information on a cost utility study that compared 3 surgical approaches of mastectomy and lumpectomy (with and without irradiation) in early-stage breast cancer surgeries in Sweden. It is one of the first studies in breast cancer to assess surgical procedures. The highlight of the study was its comprehensive approach in Markov modeling using a 6-state Markov model with 20-year hypothetical follow up (Figure 2). The results favored lumpectomy with irradiation as it appeared to be the most cost-effective option during 20-year follow-up in both healthcare and societal perspectives.
Figure 2: 6-State Markov model
Anne Marciniak, MD, PhD, MBA (Aimmune Therapeutics, UK) introduced a preliminary model that explored methods for evaluating the cost-effectiveness of first line (1L) cabozantinib/nivolumab (CaboNivo) combination therapy versus other globally available therapies for acute renal cell carcinoma (aRCC), using data available in Feb 2021. CaboNivo was recently approved as a 1L combination treatment for adults and showed a favorable cost-effectiveness profile. Marciniak highlighted that the combination generated the highest number of QALYs compared to other combinations and monotherapies.
The last speaker Chao Cai, PhD (University of South Carolina, USA) presented on the cost-effectiveness of atezolizumab plus cobimentin and vemurafenib in the treatment of BRAF V600 mutation-positive metastatic melanoma. After atezolizumab plus cobimentin and vemurafenib received FDA approval in July 2020, a question was raised regarding the cost-effectiveness of this triplet therapy. Cai’s study focused on designing a 3-state partitioned survival model to determine the triplet therapy’s cost effectiveness. The observed overall survival curves and progression-free survival curves were digitized from the IMspire150 trial. Although the cost-effectiveness of the triplet therapy exceeded the willingness-to-pay threshold, the combination can lead to significant survival benefit for patients.
While several oncology treatments options are available, a majority of them have a high price tag. Such innovative approaches are not only useful in determining the value but always helps with clinical decision making.
For more information, the full session can be viewed here: https://ispor2022.onlineeventpro.freeman.com/sessions/23883189/Cost-Effectiveness-Analysis-in-Oncology-Studies