Squaring the Circle? Leveraging Early Access/Compassionate Use Pathways to Provide Market-Specific Real-World Evidence Before Launch
Dimitrios Tzaras, MPharm; Richard Macaulay, PhD, Precision AQ, London, United Kingdom

The RWE conundrum for payers—great but too late?
Real-world evidence (RWE) is becoming an important focus area in addressing clinical trial data limitations. By leveraging RWE, manufacturers can demonstrate reproducibility of trial data in the clinical setting and alleviate potential safety and tolerability concerns. However, RWE demonstrating the effectiveness of a new therapy can typically only be generated once the product has been authorized. When it comes to reimbursement decisions, however, payers want to see product-specific RWE at launch to inform their decision making.
Could early access programs solve this RWE conundrum?
Early access or compassionate use programs (EAPs or CUPs) provide the opportunity for patients to access therapies prior to marketing authorization under certain criteria. This then allows manufacturers to generate RWE for a product in that market prior to its launch. This article aims to provide an overview of the available EAPs in 6 major European countries (Germany, France, Italy, Spain, the United Kingdom, and The Netherlands) and provide case studies of how EAP-generated clinical effectiveness RWE has been leveraged by manufacturers in HTA assessments to further substantiate their drug’s evidence package.
What EAPs exist and how do they vary?
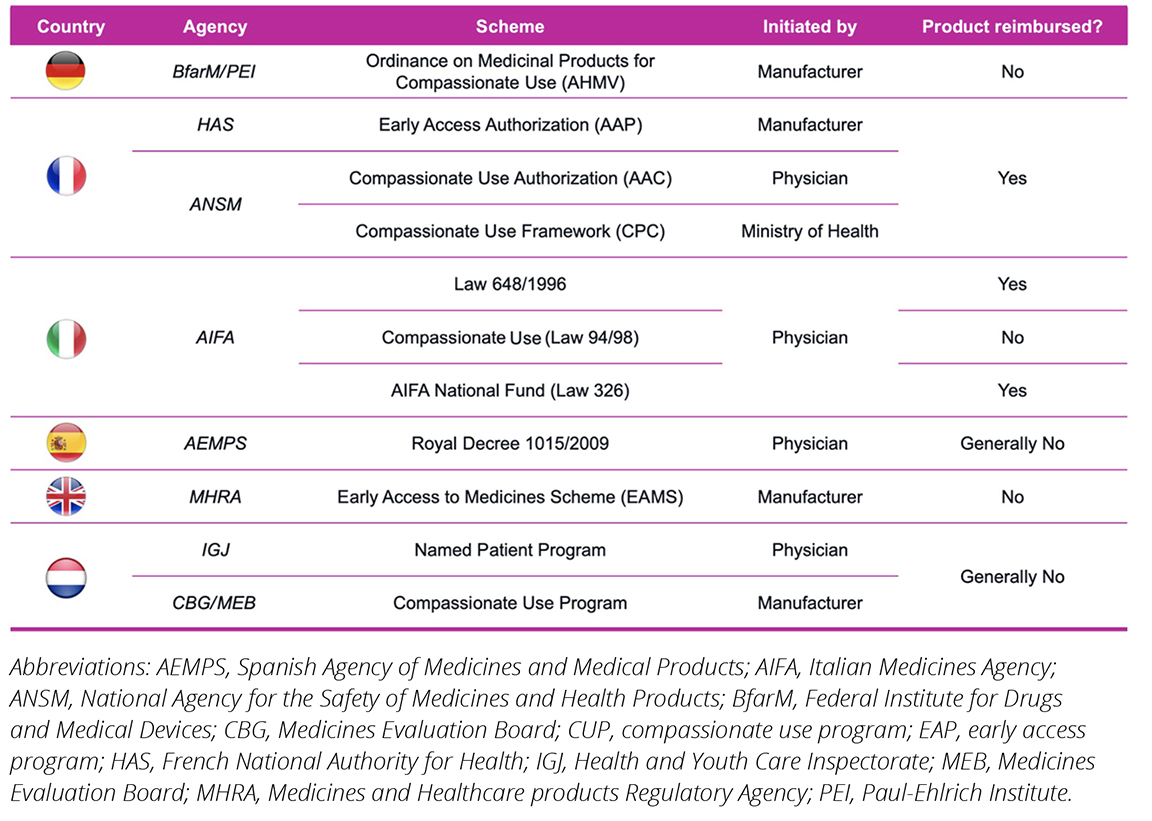
All major European countries offer EAPs through varying schemes. While some markets offer a single access pathway like Germany, Spain, and the United Kingdom, others offer multiple (France, Italy, and The Netherlands). Figure 1 outlines the available EAPs in the 6 major European markets.
As seen in Figure 1, the available EAP schemes vary per country, both in terms of which stakeholder (ie, physicians, manufacturers, or others) can initiate them and on whether they provide reimbursement incentives to manufacturers that partake in such schemes. Typically, applications can be made by either physicians or manufacturers although for some (eg, Spain) it is physician-initiation only. The overall cost of EAPs depends on reimbursement incentives provided, which vary among the countries examined. In 2/6 countries (France and Italy), full reimbursement is offered (although in Italy this is only in select programs). In another 2/6 countries (Spain and The Netherlands), reimbursement is only offered in select cases. Finally, in 2/6 countries (Germany and the United Kingdom), manufacturers must provide their treatments at zero cost. Nonetheless, these avenues provide an opportunity both for patients to access potentially life-saving medication early and for manufacturers to collect product-specific RWE outcomes prior to launch for reimbursement stakeholders.
Figure 1. Overview of EAPs/CUPs in Germany, France, Italy, Spain, the United Kingdom, and The Netherlands
Case studies—leveraging RWE from EAPs
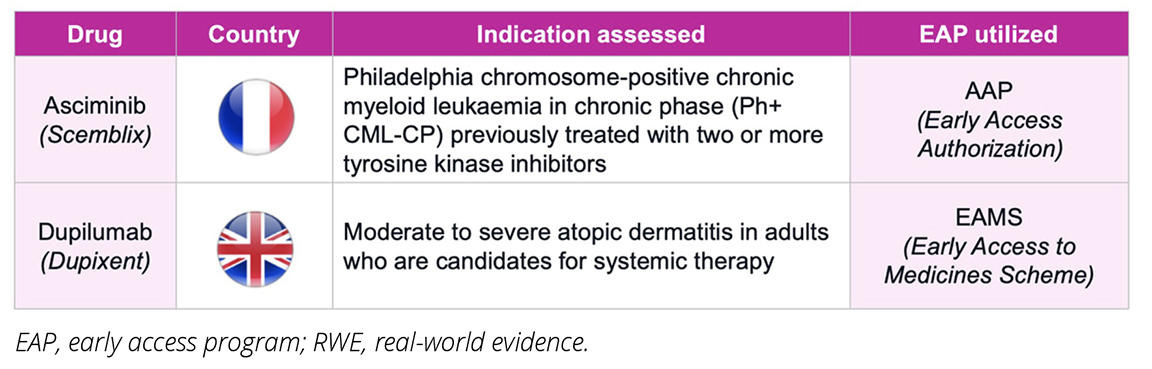
To further examine the utility and potential limitations associated with leveraging RWE from EAPs to support HTAs, we identified 2 case studies on 2 different therapies that have done this in France and the United Kingdom, asciminib and dupilumab, respectively. Figure 2 provides an overview of the aforementioned drugs, along with their respective indications and the early access schemes utilized.
Figure 2. Overview of EAP-enabled RWE collection case studies
Case study 1: Dupilumab (Dupixent)—RWE from EAPs moving the needle?
Dupilumab was granted early access via early access to medication scheme (EAMS) in March 20171 prior to its European Medicines Agency (EMA) approval in September 2017.2 It was initially appraised by the National Institute of Health and Care Excellence (NICE) in early 2018, with the initial draft guidance released on March of that year not recommending the drug on the grounds of cost-effectiveness and non-acceptable use of National Health Service (NHS) resources.3 In the subsequent consultation, supporting follow-up RWE data from the EAMS cohort were submitted, showcasing that dupilumab achieved the primary endpoint at a comparable rate in the United Kingdom’s clinical practice as in both its registrational trials.4 The supporting EAMS data also demonstrated the high unmet need for the indication, with 244 patients enrolling in the scheme in a 6-month period. Discontinuation rates were also included, which were on par with the clinical trial findings, helping support the company’s base case model. Finally, both clinicians and patients commented on the use of dupilumab via EAMS, highlighting the clinical benefits of the drug and its impact in their practice and day-to-day life, respectively. Following the final appraisal consultation, NICE overturned its initial decision, recommending dupilumab for its intended indication in August 2018.5
Case study 2: Asciminib (Scemblix)— Showcasing limitations in RWE from EAPs?
In France, asciminib was granted early access authorization for Ph+ CML-CP via the AAP scheme in April 2022,6 with its EMA approval following in August 2022.7 Data collected from the EAP were submitted for the subsequent HTA evaluation of the same indication in November 2022.8 These included information on the total number of patients that received asciminib via the AAP scheme, their dosage regime, total treatment duration, complete hematological and cytogenic response rate, and adverse event cases, including treatment discontinuations. While the RWE collected from the EAP was included in the Haute Autorité De Santé (HAS) report, it was supplementary in nature, with clear emphasis given to the pivotal trial data for the committee’s decision. The drug was eventually granted an SMR important and ASMR IV rating based on the clinical trial evidence submitted.
Early access or compassionate use programs provide the opportunity for patients to access therapies prior to marketing authorization under certain criteria.
Lessons learned and future research
The overall outcomes of these 2 case studies demonstrate the unique opportunity EAP schemes offer to manufacturers in terms of RWE collection prior to reimbursement decisions. Manufacturers can demonstrate their drugs’ effectiveness, safety, and, moreover, the reproducibility of the pivotal trial in clinical practice. The overall impact of EAP-collected RWE may vary, however, as HTA authorities use their respective methodologies in the overall hierarchy and assessment of such supporting evidence. In the case of asciminib, the manufacturer was able to utilize RWE as supplementary evidence to the drug’s clinical trial data. While the HAS report specifically mentioned the RWE collected, there was no further commentary on how these data were perceived or their overall influence in the reimbursement decision. On the contrary, the RWE submitted via the EAMS scheme in the United Kingdom was potentially key in overturning NICE’s initial reimbursement decision on dupilumab, as observed in the committee discussion commentary. The EAMS data allowed the manufacturer to both demonstrate to NICE the high unmet need of the indication and support the base case economic model. It is therefore apparent that RWE collected via EAPs can help support a drug’s value proposition, even if such evidence is qualitative in nature.
Differing perceptions of RWE highlight a key limitation in utilizing such evidence across markets that offer EAP opportunities. This may, however, change in the future as reimbursement authorities re-examine their assessment methodologies and the importance of RWE in evidence submissions. An additional challenge or barrier to this approach is the lack of reimbursement incentives, which may deter manufacturers from initiating such schemes. The observations of this article were also limited to patients with severe conditions, because EAPs are primarily offered to these patient populations. EAP methodology approaches may also pose difficulties in collecting efficacy data as they are not typically set up for this purpose. Nonetheless, the case studies presented provide an insight into the RWE opportunities that EAPs provide to generate data to support patient access. Further analysis of the impact of such evidence-collection opportunities and the differences in reimbursement outcomes of both EAP-enrolled and non–EAP-enrolled drugs will potentially provide further insights on the importance of utilizing such schemes to address clinical trial limitations and help ensure that patients can access therapies that they need.
References
- Early access to medicines scheme (EAMS) scientific opinion: Dupilumab for treatment of dermatitis. Medicines and Healthcare Products Regulatory Agency. https://www.gov.uk/government/publications/early-access-to-medicines-scheme-eams-scientific-opinion-dupilumab-for-treatment-of-dermatitis. Published March 13, 2017. Updated March 14, 2017. Accessed March 24, 2025.
- Dupixent. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent. Published November 10, 2017. Accessed March 24, 2025.
- Dupilumab for treating moderate to severe atopic dermatitis. National Institute of Health and Care Excellence (NICE). https://www.nice.org.uk/guidance/ta534/documents/committee-papers. Published April 3, 2018. Accessed March 24, 2025.
- Dupilumab for treating moderate to severe atopic dermatitis. National Institute of Health and Care Excellence (NICE). https://www.nice.org.uk/guidance/ta534/documents/final-appraisal-determination-document. Published August 1, 2018. Accessed March 24, 2025.
- Dupilumab for treating moderate to severe atopic dermatitis. National Institute of Health and Care Excellence. https://www.nice.org.uk/guidance/ta534. Published August 1, 2018. Accessed March 24, 2025.
- SCEMBLIX (asciminib) - LMC. Haute Autorité De Santé. https://www.has-sante.fr/jcms/p_3333278/en/scemblix-asciminib-lmc. Published April 22, 2022. Accessed [insert date].
- Scemblix. European Medicines Agency (EMA). https://www.ema.europa.eu/en/medicines/human/EPAR/scemblix. Published July 9, 2022. Accessed March 24, 2025.
- SCEMBLIX (asciminib) - Leucémie myéloïde chronique. Haute Autorité De Santé. https://www.has-sante.fr/jcms/p_3396012/en/scemblix-asciminib-leucemie-myeloide-chronique. Published December 16, 2022. Accessed March 24, 2025.

