Evolution of Precision Medicine: Applying a Population-Based Evidence Assessment Repository to Achieve Patient-Centered Outcomes at the Point of Care
Jonathan H. Watanabe, PharmD, PhD, University of California Irvine, School of Pharmacy & Pharmaceutical Sciences, Irvine, CA, USA; Derjung M. Tarn, MD, PhD, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA; Jan D. Hirsch, PhD, University of California Irvine, School of Pharmacy & Pharmaceutical Sciences, Irvine, CA, USA
SUMMARY
The authors propose a framework that shapes goals based on patient values and shared decision making that is continuously refined by utilizing a population-based evidence assessment repository to achieve personalized care. While the framework described will be more easily implemented in an outpatient clinic for chronic disease treatment, components could be applied to inpatient settings depending on the scenario. As more discussion and information are completed and population-derived value assessment evidence is applied, the treatment options are reduced to the most effective for the particular patient. Treatment options tailored towards the patient’s needs would be guided by the clinician’s acumen and the evidence. It would also allow the discussion to proceed based on population-based value endpoints that were then tuned based on the individual’s characteristics and wishes.
Introduction
Patient-centered care entails delivering clinical services that incorporate individual patient preferences, concerns, and needs. It ensures that values of the patient inform all treatment decisions. Patient-centered care comprises a central component of recommendations on improving the US healthcare system in the National Academy of Medicine (formerly the Institute of Medicine) seminal consensus report, “Crossing the Quality Chasm.” The goal of patient-centered care is to empower patients to be informed decision makers by providing whole-person care that is both compassionate and empathetic.1 Improvements in clinical endpoints, as well as increases in patient engagement and self-management, have been demonstrated in studies examining the impact of patient-centered care.2,3
Equal in importance to incorporating the spectrum of patient preferences in the treatment paradigm is the necessity to consider evidence that is enriched by broader, diverse study populations with analytic endpoints that are valued from the patient perspective. Juxtaposed to traditional randomized, clinical trial (RCT) data, population-based evidence sourced from real-world settings has been described as potentially more relevant, adaptable, efficient, diverse, and generalizable than RCTs. Given that traditional RCTs are generally constrained to highly specified treatment protocols for measurement of efficacy in narrow populations, large population-based, real-world studies may offer even more rich, diverse, and informative findings of how a treatment intervention will be expected to perform in actual clinical settings.4
To improve healthcare quality indicators in the United States, 2 activities must ensue. First, we must incorporate patient preferences in treatment considerations. Second, we must better apply population-based value assessments in formation of the patient care plan. To achieve the massive improvement that would move the United States closer to peer-developed nations for quality indicators, both activities must be coordinated with each other at the point of care for synergies to occur. Effectively coordinated, the process could transform healthcare by empowering patients, reducing uncertainty in clinical decision making, building efficiencies in the treatment selection process, and improving outcomes.
"The goal of patient-centered care is to empower patients to be informed decision makers by providing whole-person care that is both compassionate and empathetic."
Strengthening patient-centered care and achieving evidence-based outcomes entail bolstered application of population-derived value assessments at each step of the point-of-care visit and in follow-up care. Moreover, the massive shift to telehealth services during the COVID-19 pandemic has brought to light new challenges and new opportunities for utilizing technology to enhance patient-centered care.5 Many of these measures will likely remain after pandemic control is attained. The ability to receive additional patient-relevant information using web-based interfaces and increased adoption of secure video conference applications (eg, Zoom, Skype, Google Meet, Microsoft Teams) that facilitate sharing of important details can improve the experience for the clinician and the patient. This knowledge transmission will ultimately strengthen quality of care.
We propose a framework that shapes goals based on patient values and shared decision making that is continuously refined by utilizing a population-based evidence assessment repository (PEAR) to achieve personalized care. While the framework we describe will be more easily implemented in an outpatient clinic for chronic disease treatment, components could be applied to inpatient settings, depending on the scenario. As more discussion and information are completed and population-derived value assessment evidence is applied, the treatment options are reduced to the most effective for the particular patient (Figure 1). Treatment options tailored towards the patient’s needs would be guided by the clinician’s acumen and the evidence. It would also allow the discussion to proceed based on population-based value endpoints that were then tuned based on the individual’s characteristics and wishes.
Figure 1. Population-based evidence assessment repository (PEAR) triangle.
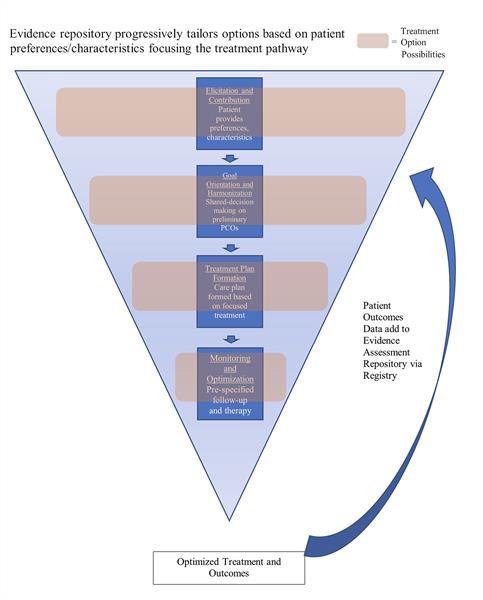
Step 1: Elicitation and Contribution
The first step we call “Elicitation and Contribution” is performed by the patient prior to the visit via internet or at patient intake via a patient portal. It involves the patient summarizing their reason for the visit to the extent possible (“elicitation”) and provides fields for the patient to “contribute” key information on their preferences, as well as social determinants, that may influence the outcomes and affect relevance of potential treatment options. As described earlier, the framework most lends itself to outpatient care where the patient has the flexibility to provide thoughtful input regarding their preferences. Along with the patient reporting the reason or chief complaint, they will describe the individual goals for the visit and the treatment plan in a text-fillable form that can aid the clinician in crafting a care plan. While many patients will report a simple short response (eg, “I want my cough to stop as soon as possible”), other patient situations would benefit from providing a more textured response (eg, “I’ve had right knee pain and my goal is to lower the pain so I can keep gardening”). This would likely entail a different treatment plan than “I’ve had right knee pain and my goal is to keep mountain climbing, so I can climb next month.” If the visit was a routine follow-up visit, there would be a reduced amount of new details in this first step of the visit. However, Step 1 still provides a structured opportunity for the patient to describe their visit goals based on their perception of their current health state.
Similar to travel agency or hotel booking websites that allow filters to be applied, the web portal would be accessible by computer, smart device, or kiosk at the point of care. It would feature a graphical user interface to capture their preferences via touch-sensitive icons, sliders, and text fields (low cost, noninjectable, side effect profile, once-daily, gender, ethnicity of provider, language). This would be stored in the electronic health record for review by the provider and the medical team at any point.
The evidence on the profound influence of social determinants of health on treatment outcomes continues to increase.6,7 While not directly causative, presence of certain demographic, social structural, and attitudinal belief factors are correlated with health services utilization.8 The proposed framework would factor in social determinants to discern options that are likely to be effective given the patient’s characteristics. The PEAR includes relevant comparative value assessments such as the National Institute for Health and Care Excellence technology appraisals and the Institute for Clinical and Economic Review assessments, and would begin sifting the evidence based on the patient’s needs, preferences, and characteristics.9,10 Based on the patient responses, the PEAR would produce an initial broad array of potential therapy options. While the clinician and the patient have yet to determine if treatment will be pursued, possible therapeutic approaches begin to materialize as options. This sets the stage for treatment to be tailored based on study type and populations in the literature to ensure relevance to the specific patient (eg, comparative effectiveness data sourced from an observational study that included older adult minorities).
Step 2: Goal Orientation and Harmonization
At the point the clinician determines that treatment is feasible and medication could remediate symptoms, the second step, “Goal Orientation and Harmonization,” occurs. Research in the field of health behavior theory has illuminated the importance of understanding the patient’s motivations and beliefs to energize behavior changes. This clarifies that patients are much more likely to adopt a behavior, embark on a treatment, or complete a treatment if the care process was commensurate with their beliefs and is consistent with their values regarding aspects such as cost, convenience, pain, and embarrassment. The objective is to “meet the patients where they are” to activate participation and performance in their own care.11
The second step involves the intentional process of determining the patient’s goals of treatment via discussion with the clinician to map to the patient-centered outcomes that are salient (“orientation”). The clinician would be supported by the PEAR to elucidate the patient-centered outcomes that are most likely to be achieved. This would foster the shared decision making needed to finalize the goals of the current care plan (“harmonization”). Incorporating the patient’s preferences and honoring the patient’s background would distill goals based on his or her priorities that were supported by evidence. The PEAR has again focused the relevant therapy options based on the goal selection.
"Assuring the patient that it is a “safe space” for them to express their concerns, needs, and beliefs is fundamental to success."
Discussing with the patient what they would like to achieve also demands level-setting on what is reasonably attainable. For example, if a patient seeks treatment after a hip fracture hospitalization and the bone-mineral density scan reveals the patient is severely osteoporotic based on a t-score of -2.5, it would be unrealistic for the patient to target a normal bone-mineral density within 1 month. The PEAR would provide outcomes information that offers an array of potential outcomes based on the patient’s characteristics and evaluation that would show the horizon of possible patient-centered outcomes and the comparative-effectiveness research studies that inform the patient and clinician on what the expected benefit could be and the uncertainty around that estimate.
This would be displayed to the clinician on the screen of a tablet or the computer in the consultation area directly from the electronic health record. The patient and the clinician would walk through the displayed outcomes from the PEAR. This would show the outcomes achieved from the possible treatments textured by the study population and type of study.
Step 3: Treatment Plan Formation
The third step is “Treatment Plan Formation.” Clinical studies are increasingly focusing on reducing medication use via trial and error approaches towards evidence-driven prescribing.12,13 To reduce uncertainty, the evidence repository would display options with the probability of success, based on the patient-centered outcomes goals laid out in Step 2 that incorporate patient preferences and characteristics elicited in Step 1. This would distill a rational pathway of initial treatments and alternative medications. This would clarify treatment options relevant to the specific patient. The evidence repository would also make clear difficulties around dosing, administration challenges (eg, need for storage upright and shaking prior to injection), and delivery complexities (eg, risk evaluation and mitigation programs, specialty pharmacy dispensing, required monitoring) that should be considered. For example, a patient with diabetes interested in reducing her hemoglobin A1C (HgbA1c) would be able to view a display of the potential medication categories that would provide their relative effectiveness averaged by group. This would allow comparison for GLP-1 agonists compared to oral DPP-4 inhibitors. Clicking on the categories would then show the within-group medications compared to each other. Medication such as a GLP-1 agonist would display its relative improvement in achieving HgbA1c compared to others within the category. The options would be perpetually filtered and reappraised based on patient preferences gleaned through the steps of the framework including benefit, patient ability to pay, complexity in administration, or dosing time. In terms of the value-based assessments, those could also be tailored based on patient preference. If the patient was only interested in medications that had demonstrated a mortality benefit, the evidence dossier would reduce to those studies and the affiliated medications. This would work similar to moving from different forest plots in a meta-analysis from the aggregated point estimate to the subgroup estimate. While the options would be prefiltered based on the information entered during Elicitation and Contribution, the system is flexible such that patient and provider could always modify the information as the discussion ensued with the PEAR responding by modifying the options.
Step 4: Monitoring and Optimization
The fourth step is “Monitoring and Optimization.” Many patients require more than 1 medication to achieve chronic disease control (eg, hypertension, diabetes) with these doses titrated to achieve maximum efficacy. “Monitoring” involves deliberate planning for follow-up based on the evidence. Effectively the treatment plan follows a longitudinal model of continuous improvement based on the follow-up findings. For example, if the treatment plan for a patient involves use of an antidepressant for improvement in depression based on improvement on the Hamilton Depression Rating Scale, then a formalized plan for follow-up visits would be scheduled based on the data regarding when the medication effect would be stabilized.14 The follow-up visit would also occur based on additional evidence including pharmacokinetic studies that inform best approaches to titrate the dose for improved effect or mitigation of side effects. As monitoring proceeds, the clinician is informed by the evidence assessment repository on data-driven steps to adjust the treatment to yield highest probability of achieving the patient-centered outcomes (“optimization”).
Patient-Centered Outcomes Experience Feeds Into a Patient Registry
The systematic longitudinal care plan guided by the evidence also provides a mechanism for the patient data to contribute to registry data that will add to evidence base. The prespecified measurement of the evidence-driven decisions and the resulting outcomes will contribute to a patient registry. This allows the framework to not only optimize care at the patient level, but also contribute to the real-world data population. This registry could then serve as a data set for studies that will contribute estimates in the PEAR at a later point. The framework not only leads to improved care for the patient receiving care but will also be harnessed to improve the quality of the real-world data.
Right Sizing the Program
Not all care settings will have the resources to accomplish all facets of the model described. Hence, evaluation will be needed of the capabilities of the clinical setting to evaluate the patient and their preferences. If care is provided at a small, rural clinic, a scaled-down application of the model would be performed. Elicitation and contribution could still be completed at or before intake. However, internet broadband capabilities may limit the ability to fully utilize the PEAR for goal orientation and harmonization. Even in this circumstance, treatment plan formation can apply the information garnered from the Elicitation and Contribution steps based on smart phone-based medication applications that detail FDA-approved outcomes. Monitoring and optimization would proceed by less technologically intensive operations. Simple discussion and mapping out of the follow-up visits, predicated on times when effect would be reached, would still allow for titration to proceed in an organized manner. Without the aid of the full population-based evidence assessment repository to guide this process based on the relevant evidence, the process is expected to involve more “trial and error” to achieve optimal medication and dose. However, consistently applying the process will facilitate improvement as technology or capacity improves in availability for clinics that are currently under resourced.
Deploying the Program
Training of clinicians would be an important first step to ensure understanding of the goal to wholly incorporate patient preferences in the process of selection of goals and treatment decisions. The model involves perpetual review of the data at each step to reshape the care plan. This refines the treatment plan based on the particular needs and wishes of the patient. A medication that is efficacious for treating high blood pressure but is associated with frequent urination in a patient who has difficulty ambulating to a bathroom on a different floor of the house would not be a wise treatment option. Ensuring that clinicians understand the overall motivation and fully appreciate the benefits from the patient perspective will be an important component of ensuring the framework functions as intended.
Incentivizing patients to participate in this new process will be achieved by clearly messaging the goal to provide better care that meets their specific needs. There will also be a diversity in patient motivation to participate in this novel care experience. Patients may be resistant, at first, to this process of eliciting their preferences, beliefs, and characteristics. Assuring the patient that it is a “safe space” for them to express their concerns, needs, and beliefs is fundamental to success.
The framework will require computational infrastructure to support the PEAR and clinician training on how to operationalize the system. Support from health information technology organizations will be a necessary component both prior to deployment and after.
Conclusion
We see this stepwise, evidence-driven, patient-centered model as the evolution of precision medicine in which therapeutic treatment is tailored beyond tissue or receptor types to therapy based on the patients’ preferences, characteristics, and their complete health state. While initial training and infrastructure are the tradeoff, the net gains in terms of improved outcomes, empathetic care, patient engagement, and operationalization of rapidly growing population-based data would easily offset the investment.
References
1. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. 2001. Washington, DC: The National Academies Press. https://doi.org/10.17226/10027.
2. Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70(4):351-379. doi:10.1177/1077558712465774.
3. Chilvers C, Dewey M, Fielding K, et al. Antidepressant drugs and generic counselling for treatment of major depression in primary care: randomised trial with patient preference arms. BMJ. 2001;322(7289):772. doi:10.1136/bmj.322.7289.772.
4. National Academies of Sciences, Engineering, and Medicine. Examining the Impact of Real-World Evidence on Medical Product Development: Proceedings of a Workshop Series. 2019. Washington, DC: The National Academies Press. https://doi.org/10.17226/25352.
5. Ting DSW, Carin L, Dzau V, Wong TY. Digital technology and COVID-19. Nat Med. 2020;26(4):459-461. doi:10.1038/s41591-020-0824-5.
6. Walker RJ, Gebregziabher M, Martin-Harris B, Egede LE. Independent effects of socioeconomic and psychological social determinants of health on self-care and outcomes in type 2 diabetes. Gen Hosp Psychiatry. 2014;36(6):662-668. doi:10.1016/j.genhosppsych.2014.06.011.
7. Quiñones AR, Talavera GA, Castañeda SF, Saha S. Interventions that reach into communities—promising directions for reducing racial and ethnic disparities in healthcare. J Racial Ethn Health Disparities. 2015;2(3):336-340. doi:10.1007/s40615-014-0078-3.
8. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 2005;83(4):Online only. doi:10.1111/j.1468-0009.2005.00428.x.
9. Pearson SD, Rawlins MD. Quality, innovation, and value for money: NICE and the British National Health Service. JAMA. 2005;294(20):2618-2622. doi:10.1001/jama.294.20.2618.
10. Pearson SD. The ICER value framework: integrating cost effectiveness and affordability in the assessment of health care value. Value Health. 2018;21(3):258-265. doi:10.1016/j.jval.2017.12.017.
11. Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2(4):328-335. doi:10.1177/109019817400200403.
12. Arango C, Kapur S, Kahn RS. Going beyond “trial-and-error” in psychiatric treatments: OPTiMiSE-ing the treatment of first episode of schizophrenia. Schizophr Bull. 2015;41(3):546-548. doi:10.1093/schbul/sbv026.
13. Leucht S, Winter-van Rossum I, Heres S, et al. The optimization of treatment and management of schizophrenia in Europe (OPTiMiSE) trial: rationale for its methodology and a review of the effectiveness of switching antipsychotics. Schizophr Bull. 2015;41(3):549-558. doi:10.1093/schbul/sbv019.
14. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. doi:10.1136/jnnp.23.1.56.

