The Comprehensive Assessment of Technologies for Child Health (CATCH) Framework: Deliberating on the Whole Child
Cindy L. Gauvreau, PhD; Avram E. Denburg, MD, PhD, Child Health Evaluative Sciences, The Hospital for Sick Children, Toronto, ON Canada

Introduction
It has long been recognized that conventional methods of health technology assessment (HTA) do not serve children and youth well, resulting in missed opportunities for health outcome improvements and diminished access to therapeutics compared to adults.1,2 Why is making HTA decisions for children more difficult and more complex than for adults? The challenges to determining the value of novel drugs for children can be broadly categorized as: 1) evidentiary limitations, particularly the paucity of randomized clinical trial data, related to difficulties in trial enrollment and the unique biology and epidemiology of childhood diseases; 2) methodological constraints of standard economic evaluation, given that techniques based on adult- or population-level beneficiaries do not estimate child-related utilities and externalities well or at all; and 3) ethical shortfalls, such as exclusionary procedures (eg, using proxies’ values rather than children’s) and persistent inequities (eg, those caused by deleterious consequences of treatments on physical and cognitive development).
However, the field of HTA is evolving in exciting ways to define value better in the new paradigms of medical research and product development.3 Child-related utility and outcomes measures are expanding.4 Consultations with patients and caregivers are becoming increasingly integrated into evaluations to complement the expert views of clinicians and economists.5 Moreover, to promote transparent and formalized (rather than intuitive) methods in complex healthcare decision making, multicriteria decision-analysis (MCDA) techniques are being considered to supplement traditional but more narrowly focused quantitative methods, such as cost-effectiveness analysis.6
In this article, we present key insights into the deliberative aspects of our research, which generated a wide range of perspectives to imbue an additive MCDA model with societal values specifically related to the health and well-being of children. The Comprehensive Assessment of Technologies for Child Health (CATCH) Framework is a child-tailored value assessment framework based on that model, operationalizing criteria deemed important to the holistic flourishing of children, beyond the immediate impacts of treatment. We apply it in the case of childhood cancer precision therapies.
This work was previously presented in parts at ISPOR Europe 2023 and in 2 peer-reviewed articles, including in the July 2024 issue of Value in Health.7-9
The CATCH Framework is a child-tailored value assessment framework based on that model, operationalizing criteria deemed important to the holistic flourishing of children.
A multistage process propelled by deliberative engagement
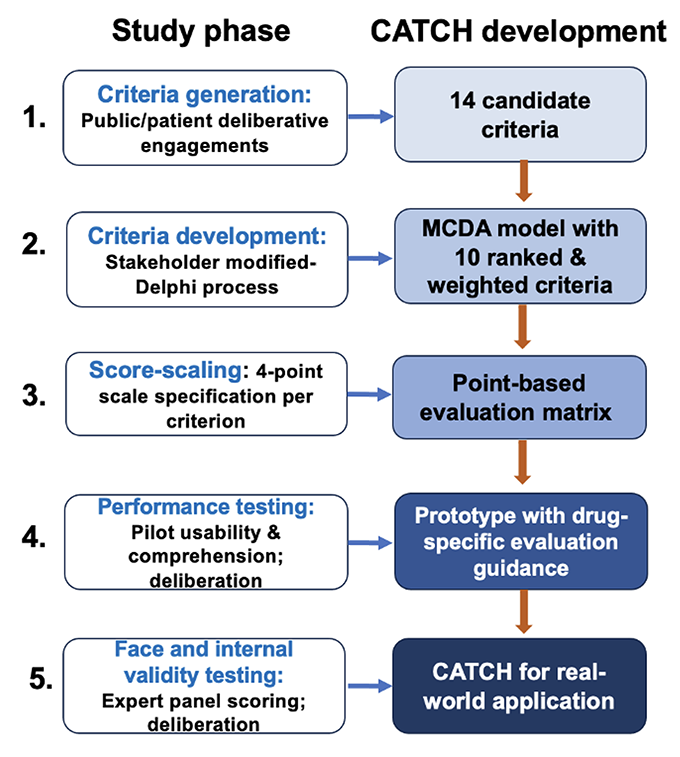
CATCH was developed through multiple iterative phases, initiated by early deliberations with the general public in “Citizen Panels,” a model of deliberative public engagement, to gather views for identifying assessment criteria. Criteria and the MCDA model were refined by a modified Delphi process and validated through 2 rounds of applied testing and deliberation (Figure 1).
Figure 1. Development of the Comprehensive Assessment of Technologies for Child Health (CATCH) Framework
Citizen views on children’s importance in society and the HTA process
The 3 Citizen Panels were broadly representative of the Canadian public but included 1 panel purposively composed of young people 16-22 years of age to gain their specific perspectives. To enable informed and meaningful deliberation, we provided technical information briefs, overviews of the Canadian drug regulatory and funding systems, and personal testimony from a caregiver with lived experience of childhood cancer. Participants thought “Childhood Distinctions,” “Voice,” “One versus Many,” and “Health System Governance” should characterize the tableau of child-tailored HTA. Within these themes, 14 candidate criteria for HTA were delineated (Figure 2).
Figure 2. CATCH Phase I themes and criteria
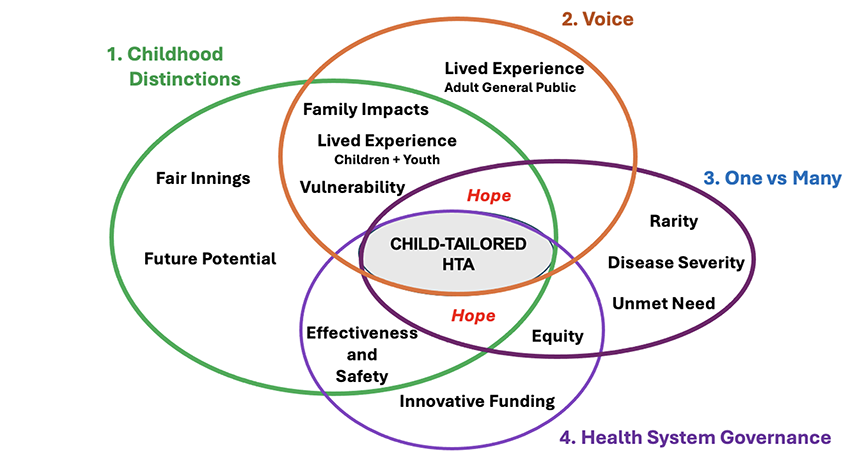
Given the transformative potential of precision drugs, it is perhaps unsurprising that Hope emerged as a pervasive subtheme (criterion), spanning multiple themes. Fair Innings, a concept stemming from health economics, was largely accepted as a valid basis of assessment, expressed by 1 participant thus, “…much as you love your grandmother, they’ve already lived up to their potential…rather than someone who is 10 years old.”
Shaping citizen values for MCDA by HTA stakeholders
Stakeholders including caregivers, health economists, bioethicists, and representatives from industry and government regulatory and reimbursement agencies were next engaged in a modified-Delphi process. Over 2 surveys and consensus-building deliberations, the broad-based and somewhat amorphous citizen values were honed and given finer-grained definitions to create criteria suitable for a MCDA model. Criteria provoking controversy were explored through group argumentation. Contrary to general-public sentiments, these participants rejected the inclusion of Hope, citing the difficulty of articulating it for HTA contexts. Fair Innings received weak endorsement due to ethical objections to the potential prioritization of younger over older populations; participants nevertheless agreed on its inclusion.
The resulting 10 criteria were considered to be nonoverlapping, important, and feasible to implement, comprising criteria that were common to HTA (Effectiveness, Therapeutic Safety, Disease Severity, Unmet Need), less common (Rarity, Equity), and, notably, new and specific to children and youth (Child-Related Health Quality-of-Life, Family Impacts, Childhood Development, Fair Innings). We allocated a budget of weights based on twice-solicited rank-order importance and quantification of “best-worst” and “discard-keep” opinions.
The wide multidisciplinarity of stakeholders, building on a foundation of public values, provided an enlarged lens uncommon to HTA processes that was nevertheless bounded by informed opinion and experience in weighing benefits and costs of health interventions. The CATCH Framework thus far described may therefore serve as a model for the role of deliberative engagement in early stage appraisal processes.
We found that members of the public and clinical and technical experts were eager to ponder and discuss the whole child and how to decide achieving the best outcomes for them.
Putting the CATCH Framework to the test
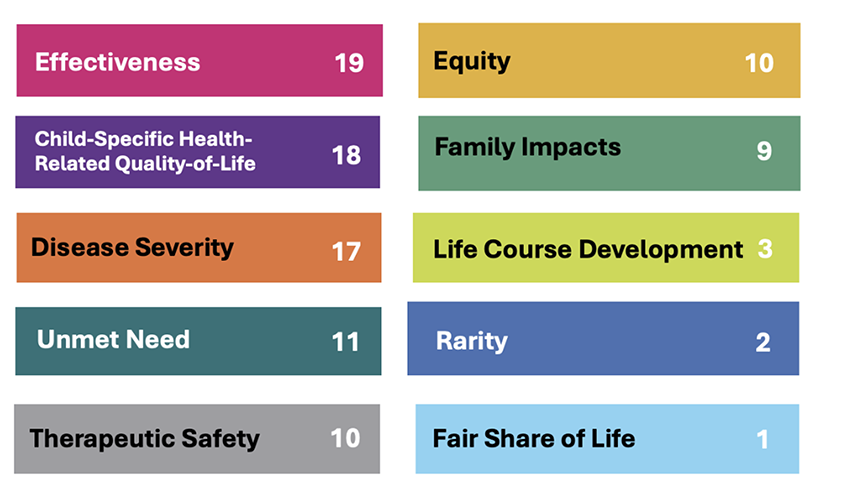
In finishing the CATCH Framework for application to appraisal, we developed a 4-point score scale for each criterion. A weighted-sum score (weight times score aggregated over 10 criteria) indicated the priority level for funding the appraised drug. Three clinicians expert in pediatric oncology drugs piloted CATCH for 10 precision drugs, by which we were able to further refine and clarify the criteria (including names) and to adjust scoring metrics and levels. In 2 deliberative rounds, Fair Innings became the more intuitively grasped Fair Share of Life, and Childhood Development became Life Course Development, better capturing unique child-specific concerns with therapies having late-effect impacts (eg, reduced fertility, secondary neoplasms). The clinicians suggested alternative scoring subcategories to capture qualitative evidence for Child-Specific Health-Related Quality-of-Life, given a typical lack of quantitative evidence, and to distinguish between additive and substitutive therapies in Therapeutic Safety. Figure 3 shows the final set of criteria and their respective weights.
Figure 3. Final CATCH criteria and weights
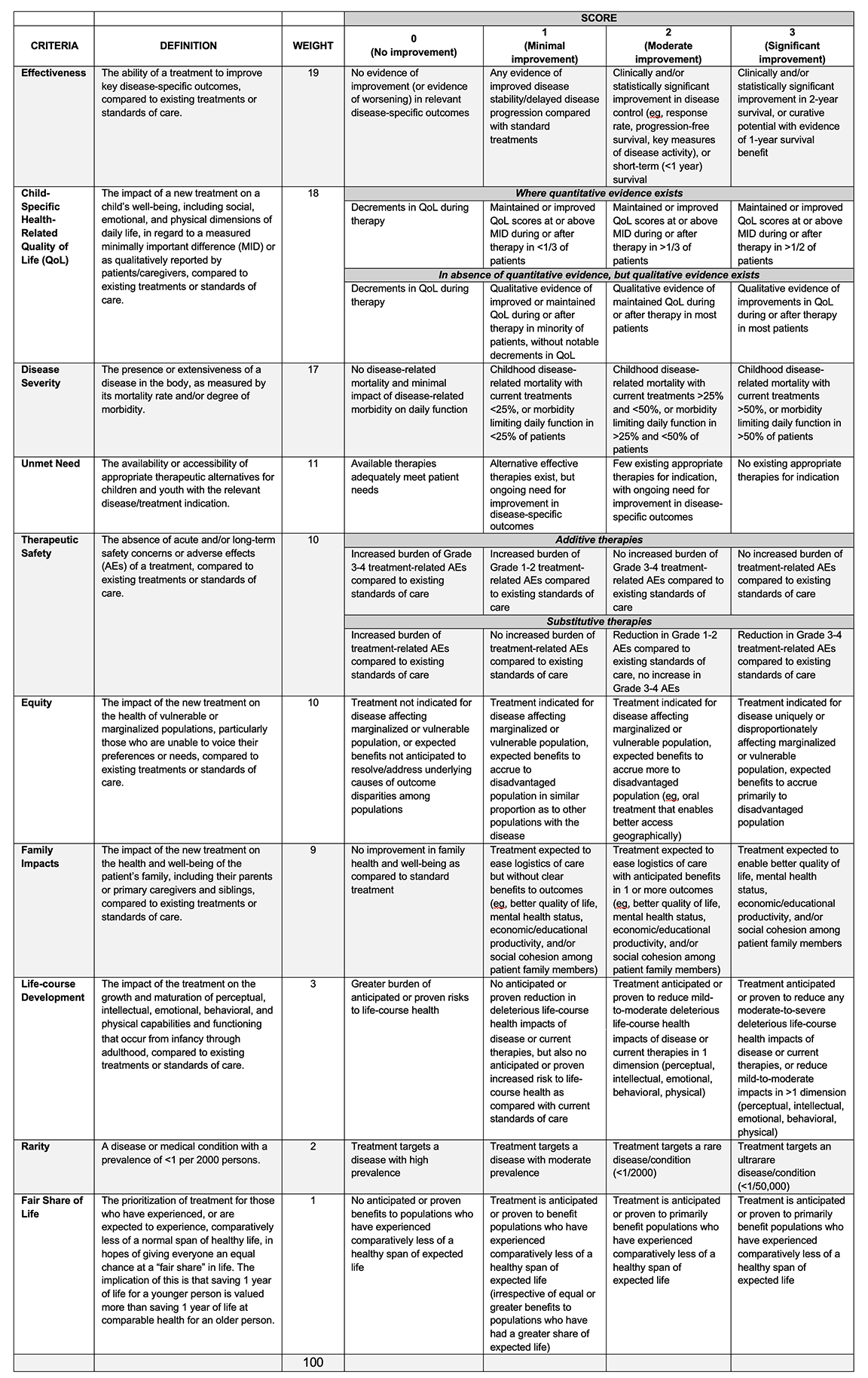
The refined CATCH Framework, Table 1, was then validated by a group of pediatric oncologists and a pharmacist in a mock HTA appraisal committee. Participants were provided with CATCH and clinical evidence briefs for blinatumomab (indicated for relapsed B-cell acute lymphoblastic leukemia) and brentuximab vedotin (high-risk Hodgkin’s lymphoma), accompanied by guidance on relating the individual evidence components to specific criteria and scoring levels. Participants scored individually, then the group was convened to share their rationales and to arrive at consensus scores and, consequently, funding recommendations. The group affirmed that in their expert opinions, the novel, child-specific criteria were relevant and meaningful for assessing the 2 targeted therapies on important health and well-being outcomes. Furthermore, they affirmed the coherence of the standard and child-specific criteria together within the CATCH Framework.
We completed our study by varying the weights of the child-specific criteria to examine the impact on the weighted-sum scores. As there were no resulting significant shifts in funding recommendations, we concluded that CATCH was robust for application to childhood cancers. The intrinsic benefit of CATCH for application to other childhood conditions is, we believe, the early and explicit focus on values for child health and well-being, sustained through iterative, progressive deliberation with as wide a stakeholder perspective as possible.
Table 1. The Comprehensive Assessment of Technologies for Child Health (CATCH) Framework
Implications for HTA organizations
In the current environment of HTA evolution, there is an opportunity to consider evaluation methods that not only embrace wider notions of value in child health technologies but also increase decisional legitimacy. We found that members of the public and clinical and technical experts were eager to ponder and discuss the whole child and how to decide achieving the best outcomes for them. For example, participants expressed (clinical) Effectiveness as the premier criterion (weight = 19, out of 100 points), thus affirming the legitimacy of HTA agencies standardly using this criterion as a “first hurdle.” Importantly, Child-Specific Health-Related Quality of Life ranked second (weight = 18), over another standard criterion, Therapeutic Safety, indicating participants’ high regard for the child’s entire treatment experience.
In the CATCH Framework, we have created a structure and heuristic to strengthen and develop deliberative engagement in HTA assessment of child health technologies. It delineates a role for stakeholder participants, including those who do not have an immediate stake in HTA decisions, to provide societal values and to identify assessment criteria for which evidence must be gathered and debated during decision making, furthering the international development of evidence-informed HTA deliberation.10
The challenge remains to create a more generalized version of CATCH that could be applied to other childhood conditions. We are currently working toward this goal. Ultimately, we aim to fill a gap in evaluating child health technologies by incorporating a child-tailored value assessment framework based on CATCH in HTA processes in Canada and internationally.
References
- Ungar WJ, Prosser LA, Burnett HF. Values and evidence colliding: health technology assessment in child health. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):417-419. doi:10.1586/14737167.2013.815420
- Denburg AE, Giacomini M, Ungar W, Abelson J. Ethical and social values for paediatric health technology assessment and drug policy. Int J Health Policy Manag. 2022;11(3):374-382. doi:10.34172/ijhpm.2020.144
- O’Rourke B, Oortwijn W, Schuller T. The new definition of health technology assessment: a milestone in international collaboration. Int J Technol Assess Health Care. 2020;36(3):187-190. doi:10.1017/s0266462320000215
- Rowen D, Rivero-Arias O, Devlin
N, Ratcliffe J. Review of valuation methods of preference-based measures of
health for economic evaluation in child and adolescent populations: where are
we now and where
are we going? Pharmacoeconomics. 2020;38(4):325-340. doi:10.1007/s40273-019-00873-7 - Oortwijn W, Husereau D, Abelson J, et al. Designing and implementing deliberative processes for health technology assessment: a Good Practices Report of a joint HTAi/ISPOR Task Force. Value Health. 2022;25(6):869-886. doi:10.1016/j.jval.2022.03.018
- Baltussen R, Marsh K, Thokala P, et al. Multicriteria decision analysis to support health technology assessment agencies: benefits, limitations, and the way forward. Value Health. 2019;22(11):1283-1288. doi:10.1016/j.jval.2019.06.014
- Gauvreau CL, Schreyer L, Gibson P, et al. Widening the value lens for child health technologies: development of a child-tailored HTA value assessment framework using multi-criteria decision analysis incorporating patient, public, and expert perspectives. Value Health. 2023;26(12):S351. doi:10.1016/j.jval.2023.09.1852
- Gauvreau CL, Wight L, Subasri M, et al. Access to novel drugs and therapeutics for children and youth: eliciting citizens’ values to inform public funding decisions. Health Expect. 2023;26(2):715-727. doi:10.1111/hex.13697
- Gauvreau CL, Schreyer L, Gibson PJ, et al. Development of a value assessment framework for pediatric health technologies using multicriteria decision analysis: expanding the value lens for funding decision making. Value Health. 2024;27(7):879-888. doi:10.1016/j.jval.2024.03.012
- Oortwijn W, Jansen M, Baltussen R. Use of evidence-informed deliberative processes by health technology assessment agencies around the globe. Int J Health Policy Manag. 2020;9(1):27-33. doi:10.15171/ijhpm.2019.72

