Fast-Tracking Digital Health: How Accelerated Coverage Pathways for Innovation Are Paving the Way for Innovation
Anjali Ramkeesoon, BSc (Hons), MSc, Alira Health, London, United Kingdom; Ons Ben Dhia, MSc, PharmD, Alira Health, Paris, France
.png?sfvrsn=4d86ddfc_1) The Future of Digital Health Adoption
The Future of Digital Health Adoption
The rapid rise of digital health technologies (DHTs), such as therapeutic digital solutions and remote patient monitoring solutions, is reshaping healthcare, personalizing treatment, expanding access, and improving patient outcomes. However, despite their potential, these innovations often struggle to fit within traditional reimbursement structures, creating barriers to widespread adoption. To bridge this gap, several countries have introduced accelerated coverage pathways for innovation (ACPIs), designed to fast-track market access for cutting-edge healthcare solutions.
ACPIs function as early access programs, facilitating the reimbursement and integration of emerging medical technologies, including DHTs, in vitro diagnostics (IVDs), and novel therapeutics.1 By addressing clinical and economic uncertainties, these pathways support healthcare providers in delivering innovative solutions to patients faster and more efficiently.
This article explores how leading ACPIs across Europe, such as the United Kingdom’s AI in Health and Care Award, Germany’s DiGA, and France’s PECAN, are shaping the digital health landscape, their impact on market access, and the challenges that remain in ensuring sustainable adoption.
Accelerated coverage pathways for innovation offer significant benefits to patients by expediting access to groundbreaking therapies and diagnostics.
The Market Access Challenge: Why DHTs Need ACPIs
Despite the promise of DHTs, their integration into healthcare systems remains an uphill battle. Unlike traditional medical devices, DHTs face several challenges, such as regulatory uncertainty, interoperability issues, and stringent data privacy requirements. The fast-paced nature of digital innovation only adds to the challenge; by the time a technology clears reimbursement hurdles, it risks becoming obsolete (Figure 1).
Figure 1. Graphic demonstrating the key challenges faced by digital health technologies for integration in various health systems.
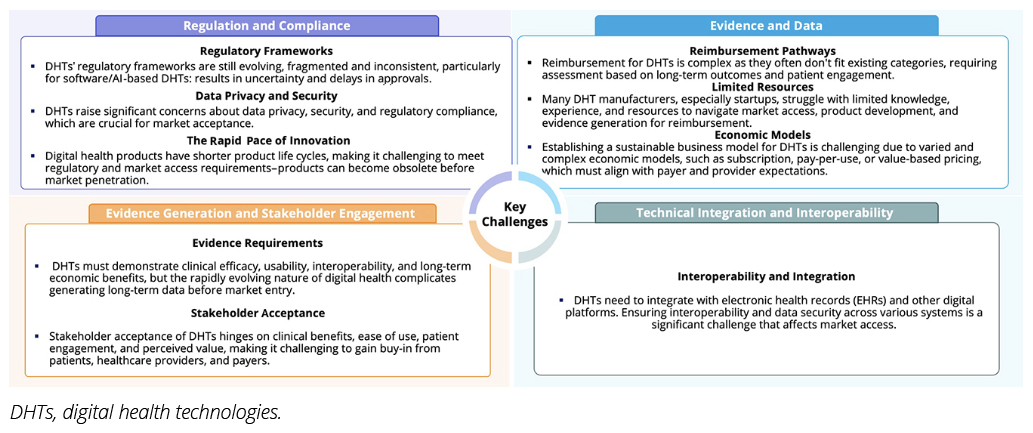
These complexities underscore the necessity of ACPIs. Early access pathways are tailored to DHTs, as they are designed to provide structured yet adaptable solutions, offering a lifeline for cutting-edge digital health solutions seeking market entry. By streamlining reimbursement and regulatory processes, these pathways ensure that groundbreaking technologies don’t just remain theoretical advancements but reach the patients who need them most.
These pathways provide temporary funding for solutions that are not yet mature enough for long-term reimbursement and require additional evidence generation. They are generally selective, with varying eligibility criteria based on factors like development stage and CE marking.
Fast-Track to the Future: How Europe Is Funding DHTs
When it comes to accelerating the adoption of digital health technologies, Germany, France, and the United Kingdom (UK) are leading the way.
Digital Health Applications (DiGA, Germany) focuses on low-risk digital health applications (Class I or II under European Medical Device Registration), allowing 1 year of manufacturer-set reimbursement while companies gather clinical and economic evidence to demonstrate positive healthcare effects for permanent listing.4,5
La prise en charge anticipée numérique (PECAN, France) has a broader eligibility scope, covering remote patient monitoring and higher-risk medical devices (Class I-III). It provides temporary coverage (set by the Ministry) for 1 year, giving manufacturers time to generate the necessary clinical and economic data for permanent reimbursement.2
Artificial Intelligence (AI) in Health and Care Award (UK) directly funds technologies across multiple development phases, from feasibility studies to large-scale adoption. It also supports devices with market approval (MA) that require further evidence for full integration into the healthcare system.3
Each pathway serves as a launchpad for innovation, enabling manufacturers to secure temporary coverage and build the necessary evidence base for long-term reimbursement and adoption.
A Closer Look at Europe’s Leading ACPIs
A comparative analysis of 3 major digital health–focused ACPIs (AI in Health and Care Award [UK], DiGA [Germany], and PECAN [France]) reveals their unique approaches to facilitating DHT adoption (Figure 2).
Figure 2. The 3 accelerated coverage pathways for innovation used in this analysis. They were chosen based on their pathway designs for digital health technologies adoption in EU5 health systems.
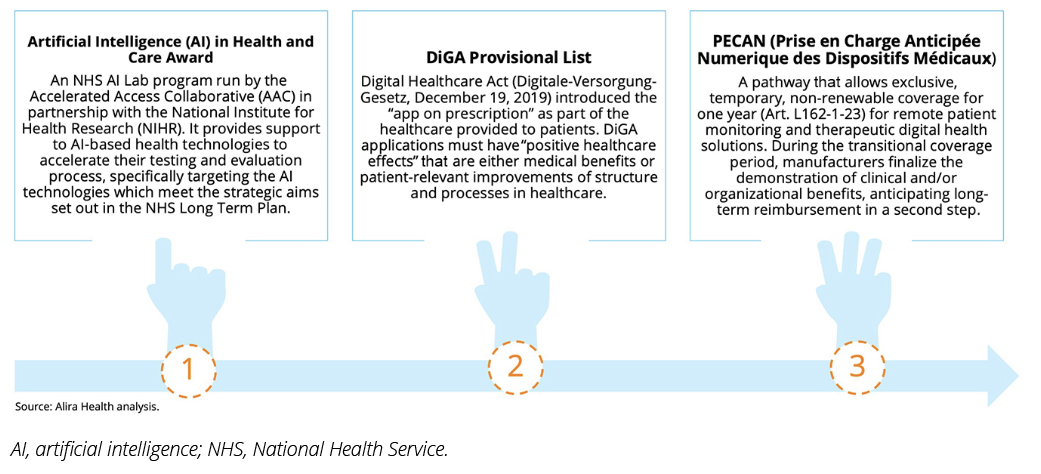
While all innovative pathways for DHT adoption in the 3 countries aim to expedite the delivery of potentially life-saving technology, each targets different types of solutions and stages of development.
- The UK’s AI in Health and Care Award demonstrated the highest theoretical eligibility (100%), followed by Germany and France (50% each). This suggests that the UK’s system is the most accommodating for DHTs at various development stages.
- Unlike AI in Health and PECAN, Germany’s DiGA pathway does not strongly emphasize remote patient monitoring (RPM), potentially limiting the adoption of RPM-dependent solutions. However, with AI-driven RPM gaining traction (eg, RPM for diabetes patients, oncology patients, and in patients with chronic diseases in general), future adjustments to DiGA may integrate more such technologies.
Overall, the eligibility criteria are similar across the 3 pathways. The main differences would be in the requirements of CE marking and the integration of AI (Table 1). The PECAN pathway is still evolving, with only 1 device assessed under the program at the time of this analysis.
Table 1. Applicability of the 2 digital health technologies analyzed on the eligibility criteria of the 3 accelerated coverage pathways for innovation.
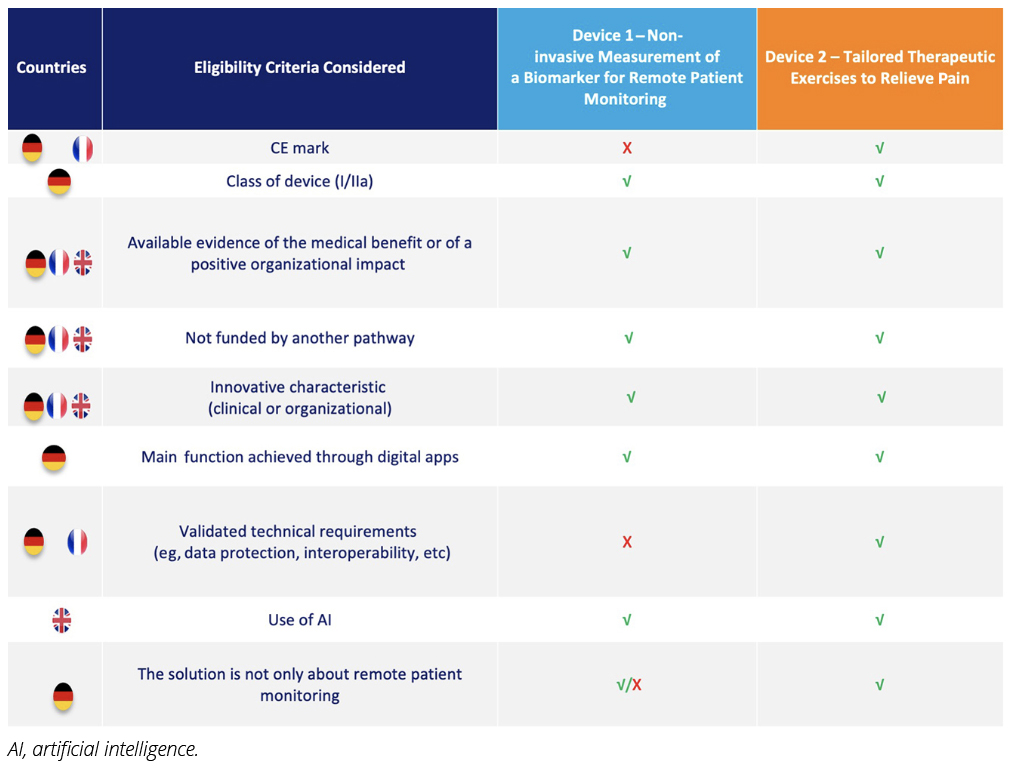
Pathway Perspectives: The Manufacturer’s Dilemma
For manufacturers of DHTs, securing reimbursement is often as complex as developing the technology itself. Success hinges on meeting rigorous clinical and economic benchmarks, navigating country-specific regulations on safety, AI, and data privacy, and crafting a market strategy that aligns with evolving funding opportunities. But with each country applying its own rules, the landscape is anything but uniform.
Germany, for example, enforces strict safety and operability requirements, particularly for later-stage therapeutic devices, while the UK takes a more flexible approach, focusing on earlier-stage AI-driven innovations without mandating a CE mark. Programs like the AI in Health and Care Award stand out by offering comprehensive support, funding, National Health Service integration, and real-world evidence generation, helping manufacturers bridge the gap from development to adoption. Meanwhile, PECAN in France and Provisional DiGA in Germany provide temporary funding, allowing companies to introduce their products while refining clinical evidence. These pathways don’t just lower financial risk for manufacturers; they also accelerate patient access to potentially life-changing technologies.
Policy makers hold the key to making accelerated coverage pathways for innovation a driving force in healthcare innovation.
Fast-Track Access: A Win for Patients
Beyond industry stakeholders, ACPIs offer significant benefits to patients by expediting access to groundbreaking therapies and diagnostics. Programs like the AI in Health Award involve patients in early stage real-world evidence generation, ensuring that life-changing technologies reach them sooner.
Similarly, DiGA and PECAN provide transitional coverage, allowing patients to access treatments while long-term reimbursement decisions are pending. In Germany, the Provisional DiGA model enables manufacturers to set interim prices—generating revenue while collecting additional clinical data. Meanwhile, PECAN serves as a bridge to France’s broader reimbursement system, facilitating faster integration into standard care.
These 3 programs enable patients to access the technology earlier, offering quicker relief and improved outcomes for those who need it most.
Policy Implications: Driving Healthcare Transformation
Policy makers hold the key to making ACPIs a driving force in healthcare innovation. By streamlining reimbursement processes, they don’t just accelerate the adoption of DHTs, they also create a ripple effect across the entire healthcare ecosystem. More efficient coverage pathways lead to long-term cost savings, particularly in managing chronic diseases, where early intervention can prevent expensive complications. At the same time, automation and AI-driven solutions free up critical healthcare resources, allowing professionals to focus on complex cases rather than administrative burdens.
Beyond immediate efficiencies, ACPIs also fuel the growth of the digital health sector, encouraging investment in emerging technologies that have the potential to redefine patient care. A well-structured ACPI strategy doesn’t just support innovation, it future-proofs healthcare systems, ensuring they remain adaptable, responsive, and ready for the next wave of technological advancements.
Looking Ahead: The Future of ACPIs
DHTs present a unique challenge, balancing rapid innovation with regulatory rigor. While ACPIs provide an essential bridge for early adoption, their long-term impact remains uncertain due to selective eligibility and temporary coverage models. As healthcare systems mature, more permanent and streamlined assessment processes will likely emerge.
Ultimately, manufacturers, patients, and policy makers must collaborate to refine these frameworks, ensuring that digital health innovations not only reach the market but also sustain long-term impact in patient care.
References:
- MedTech Europe. From Diagnosis to Cure: Facts & Figures Report. http://www.medtecheurope.org/wp-content/uploads/2024/07/medtech-europe--facts-figures-2024.pdf. Published 2022. Accessed January 2024.
- Haute Autorité de Santé (HAS). Early reimbursement of a digital medical device (Art. L.162-1-23 of the CSS). https://has-sante.fr/upload/docs/application/pdf/2023-03/pecan_guide_de_depot_de_dossier.pdf. Updated October 31, 2024. Accessed February 2024.
- National Institute for Health and Care Research (NIHR). Artificial Intelligence in Health and Care Award - Guidance for Competition 3 All Phases. https://www.nihr.ac.uk/artificial-intelligence-health-and-care-award-guidance-competition-3-all-phases. Published October 30, 2020. Updated June 2021. Accessed March 2023.
- Federal Institute for Drugs and Medical Devices. The Fast-Track Process for Digital Health Applications (DIGA) according to Section 139e SGBV. https://www.bfarm.de/SharedDocs/Downloads/EN/MedicalDevices/DiGA_Guide.html. Published August 7, 2020. Accessed March 2024.
- MTRC Research. Mapping the Pathways Enabling Market Access to Innovative Medical Procedures and Technologies. https://argeokulu.bogazici.edu.tr/sites/argeokulu.bogazici.edu.tr/files/innovative_payment_schemes_report.pdf. Published 2022. Accessed March 2024.

