Are Digital Therapeutics Reshaping the Health Technology Assessment Framework for Health Technologies?
Lucia Pérez-Kempner, Pharm, MSc, MBS, Parexel International, Madrid, Spain; Elisa Sain, MSc, Parexel International, Bucharest, Romania; Malgorzata Bieniek, MSc, Parexel International, Krakow, Poland
 The role of patient insights in HTA decision making has historically been limited
The role of patient insights in HTA decision making has historically been limited
Health technology assessment (HTA) is a comprehensive process that evaluates the clinical benefits and economic implications of a health technology through evidence-based research and quantitative analysis. When assessing health technologies, the evaluation of these benefits has traditionally considered the disease background, unmet need, and the clinical and economic value of the product. In situations where the value is weighted against costs, economic evaluations are used to improve the rational and efficient use of financial resources, with the coverage of technologies remaining subject to their cost-effectiveness and affordability in many markets.1
In the last few years, the incorporation of the humanistic value of health technologies into the HTA discussion has increased. Elements related to the patient’s value are captured through patient-reported outcomes (PROs) in clinical trials, surveys, or studies involving patient inputs. However, these elements tend to impact decision making only when there is high uncertainty or incomplete evidence, or when PRO data are required to build economic models. Therefore, several important patient benefits (such as patients’ preference, convenience, symptom experience, treatment satisfaction, etc) may be overlooked in the current HTA frameworks.
The Food and Drug Administration (FDA) has defined digital health technologies (DHTs) as “systems that use computing platforms, connectivity, software, and/or sensors for healthcare and related uses.”2 Examples involve mobile applications, such as CANKADO, which provides patient support, patient diaries, and documentation on PROs for patients suffering from HR+ HER2- metastatic breast cancer. The emergence of DHTs has raised a need to conduct HTAs on these technologies. Several HTA agencies have changed or adjusted their traditional assessment frameworks to adapt to the new specificities of DHTs. Examples include the Gemeinsamer Bundesausschuss (G-BA) in Germany, the National Institute for Health and Care Excellence (NICE) in the United Kingdom (UK), and the Haute Autorité de Santé (HAS) in France.3 Given the critical link between the health outcomes of DHTs and patients’ ability to use the DHTs, there has been a growing awareness of patients’ experience with the use of DHTs and of the impact that this has on the safety and efficacy of the DHTs. Consequently, the HTA frameworks of several HTA agencies have been adjusted to further increase the degree at which patient experience is incorporated into the HTA decision-making process.
"We explore how the increase in the “voice of the patient” in digital health technology assessment is reflected in the evaluations and how it may shape future HTA processes for drugs."
Understanding the ongoing shifting paradigm of the use of patient insights is critical for identifying current trends that may shape the future of HTA decision making
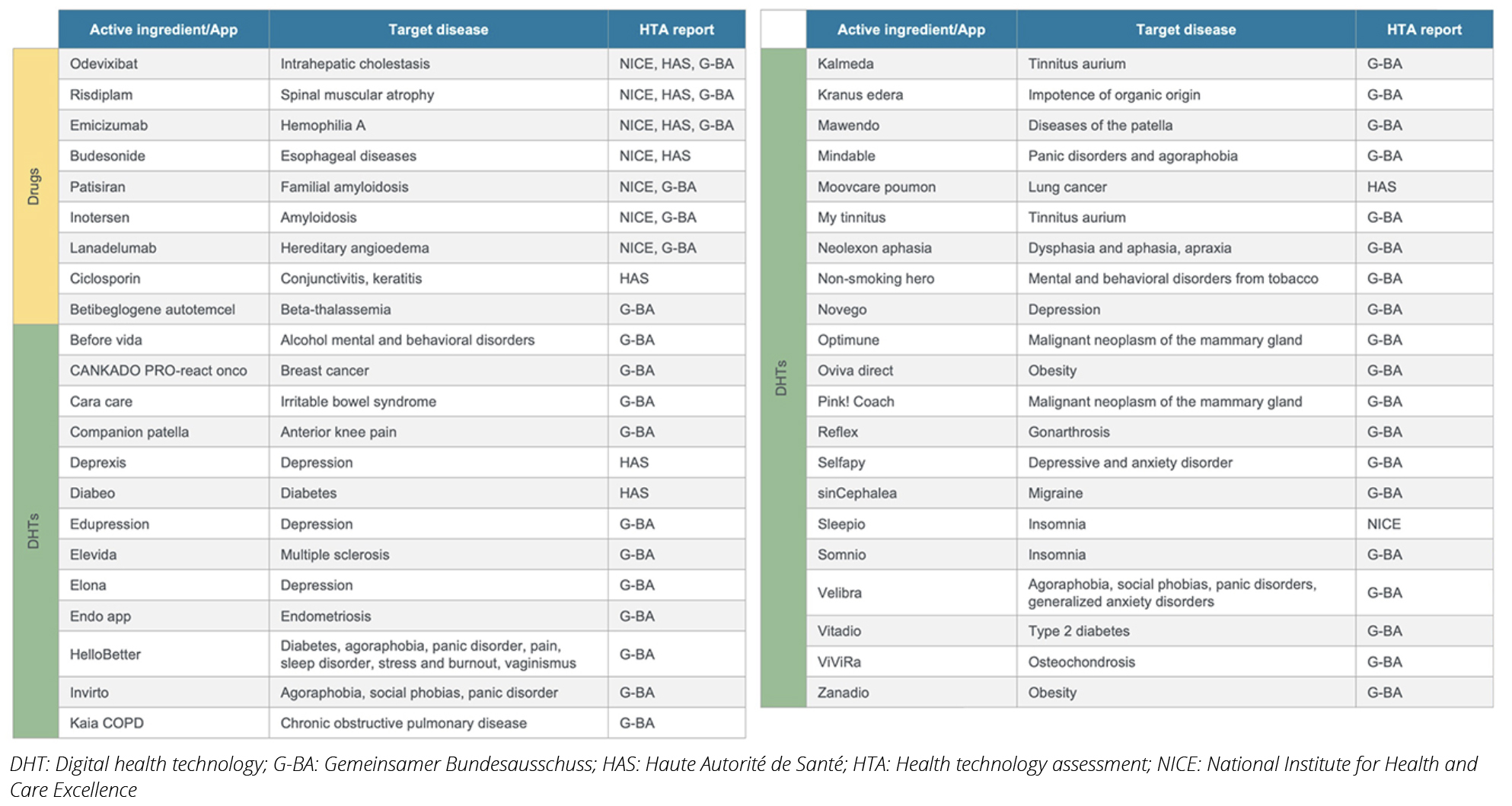
The recent emergence of DHTs and the need to establish an HTA framework in this area have led HTA decision makers to pay more attention to PROs. In this article, we explored how the increase in “voice of the patient” in DHT assessment is reflected in the evaluations and how it may shape future HTA processes for drugs. To identify innovative drugs in Europe, we searched the European Medicines Agency (EMA) database for drugs approved via an accelerated pathway between 2018-2022. Drugs approved via the accelerated pathway by EMA capture drugs with the greatest need for access. Nine drugs in various therapeutic indications were identified. The HTA reports for these drugs were retrieved from the G-BA, NICE, and HAS websites (Table 1). We also identified DHTs assessed by these agencies up to 2022, noting that DHTs are approved by each country’s regulatory agency, unlike the centralized process for drugs. (Table 1). For both innovative drugs and DHTs, we analyzed HTA reports, focusing on patient-related insights and PRO data, including quality of life (QoL) information. Using thematic analysis, we assessed which patient insights were considered in these reports and how often. Finally, we examined the rationale behind HTA decisions to identify patient-related factors that were important determinants for the HTA decisions.
Table 1. Drugs and digital health technologies identified and analyzed
The impact of patient insights on HTA decision making for drugs remains low, with PRO data being increasingly used but having limited influence on the final HTA decision
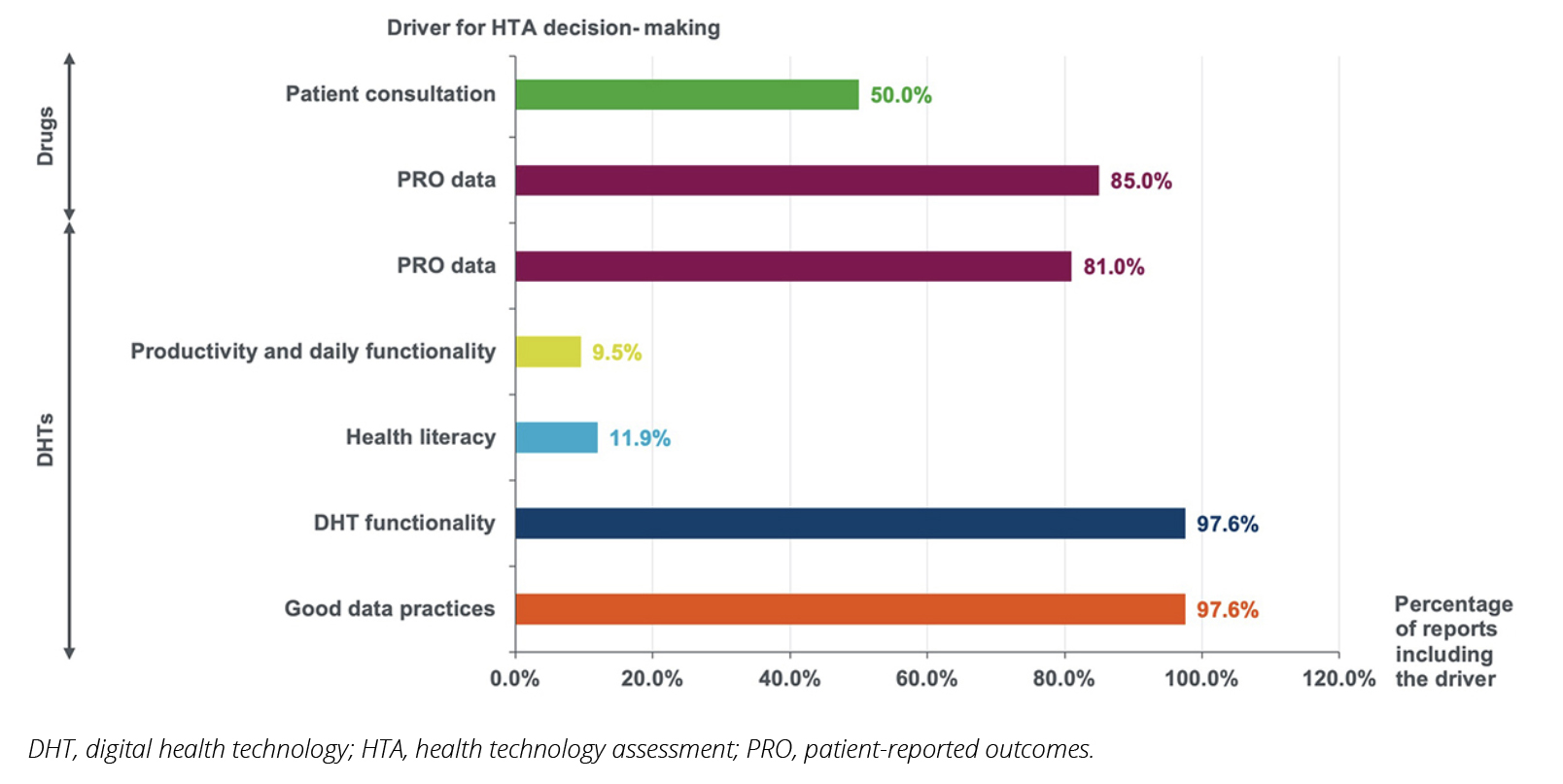
Our analysis of HTA decisions for both drugs and DHTs reveals interesting trends in how patient perspectives are considered across different technology types. Among the 20 HTA reports identified with published decisions for drugs, 50% and 85% included patient consultation and PRO data, respectively (Figure 1). While the inclusion of PRO data in the evaluation was high (85%), it was also evenly distributed across the 3 HTA agencies; in contrast, patient consultations were conducted in 50% of the evaluations by NICE and HAS only. When analyzing the impact of patient consultation and PRO data on the final HTA decision, HAS appeared to give significant weight to those factors, while NICE and G-BA seemed to place somewhat less emphasis on them (Figure 2). As expected, clinical benefit remained the primary factor in the HTA decisions made, with cost-effectiveness also being an influencing factor, particularly in the United Kingdom.
Figure 1. Drivers of health technology assessment decision making identified through health technology assessment appraisals
In contrast with the HTA framework for drugs, patient insights are a main driver for HTA decision making when evaluating DHTs
When it comes to DHTs, we observed a substantial shift in approach by HTA agencies compared to the evaluation of drugs. Patient insights emerged as a crucial driver in HTA decision making for these technologies. The majority of DHT assessments included PRO data (81%) (Figure 1). Moreover, new patient-relevant elements were included, specifically the assessment of increases in patients’ productivity and daily functioning as well as their ability to understand and use the technology effectively (health literacy). While not directly related to patient experiences, DHT functionality and good data practices were almost universally considered in assessments. These factors indirectly reflect patients’ ability and willingness to use the technologies, further emphasizing the patient-centric approach in DHT evaluations. Overall, 98% of analyzed HTAs for DHTs included elements that either directly and/or indirectly considered patient-relevant drivers for decision making (Figure 1).
Germany’s G-BA showed the most diverse range of considerations in their DHT assessments, likely due to the country’s pioneering efforts in advancing access to these technologies. NICE and HAS consistently emphasized DHT functionality and data practices in their evaluations.
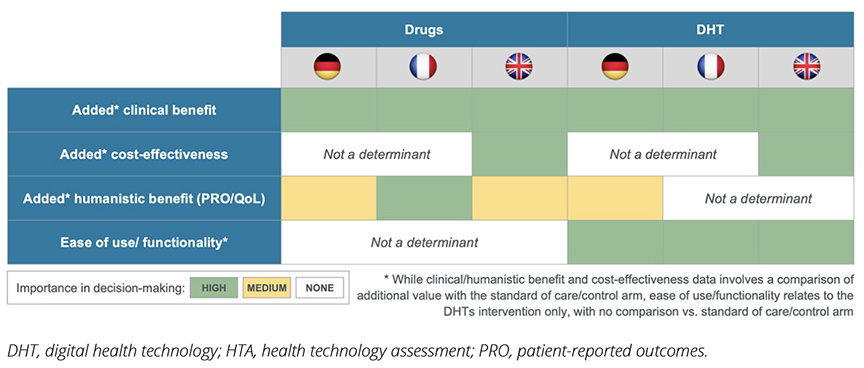
Overall, similar to the evaluation of drugs, clinical benefit remains the primary factor in the HTA decision making for DHTs. However, a notable distinction emerges in the evaluation of DHTs: ease of use and functionality are consistently strong determinants in decision making across all 3 countries studied. This contrasts with drug assessments, where these factors play no role at all (Figure 2). This difference may highlight a new trend: the evaluation of DHTs is evolving toward a more patient-centric approach, diverging from the traditional assessment methods used for pharmaceuticals.
Figure 2. Comparisons of the influence of different drivers in health technology assessment decision making
The way forward—placing the patient at the heart of the HTA decision making
While patient insights remain as an optional component of clinical trials, health stakeholders are advocating for the inclusion of these insights, going beyond the traditional QoL metrics commonly included. This aims to elevate the role of patients in the research and development (R&D) process of health technologies, with subsequent implications on the decision making for their marketing authorization and financing. As the R&D of DHTs increases, with subsequent increases in the number of DHTs launched and requesting funding, the number of clinical trials that place patients’ ability to use and understand health technologies and the importance of adherence and compliance in direct relation to clinical outcomes and the value of health technologies increases.
When it comes to DHTs, we observed a substantial shift in approach by HTA agencies compared to the evaluation of drugs. Patient insights emerged as a crucial driver in HTA decision making for these technologies.
The ongoing increase in HTA appraisals for DHTs will likely boost the number of evidence packages that focus not only on traditional disease, clinical, and economic value arguments but also on patient insights and their understanding of the technology and the value of compliance/adherence. Furthermore, patients’ transition from being passive recipients of care to valuable sources of data to support the value of products has the potential of empowering patients to become more active in their participation in decision-making processes that involve their own health. Patient-centered evidence packages, in combination with patient empowerment, may drive advocacy efforts to increase the role that patient insights play across all levels of access to health technologies. This includes patient participation at the HTA decision making level through participation in HTA consultation processes. This will likely be further supported by existing processes in some countries, such as Canada and the UK, where patient consultation is already part of the HTA decision-making process.
Nonetheless, quantifying patient insights through robust metrics can be challenging, particularly in vulnerable populations (eg, children, disabled people). Furthermore, the incorporation and/or amendment of new and/or existing guidelines, policies, and/or regulations defining the frameworks for HTA decision-making processes can be time consuming, with governments lacking the resources required. Methodological variability across and within countries may further increase the challenges for the adoption of patient insights within countries’ HTA frameworks.
Collaboration among all providers, recipients of care, industry, and policy makers is important to foster efforts to advance patient insights in the HTA decision-making process. Governments should perceive the allocation of resources to support these efforts as an investment that can maximize the efficiency of the HTA decision-making process. Moreover, the industry should consider switching traditional R&D processes to incorporate patient insights earlier in the clinical development program.
References
- Bodrogi J, Kaló Z. Principles of pharmacoeconomics and their impact on strategic imperatives of pharmaceutical research and development. Br J Pharmacol. 2010;159(7):1367-1373. doi:10.1111/j.1476-5381.2009.00550.x
- Framework for the Use of Digital Health Technologies in Drug and Biological Product Development.. US Food & Drug Administration. https://www.fda.gov/science-research/science-and-research-special-topics/digital-health-technologies-dhts-drug-development#framework. Published March 2023. Accessed August 15, 2025.
- Mezei F, Horváth K, Pálfi M, Lovas K, Ádám I, Túri G. International practices in health technology assessment and public financing of digital health technologies: recommendations for Hungary. Front Public Health. 2023;11:1197949. doi:10.3389/fpubh.2023.1197949

