A Case of Multitasking: Conducting and Using HTA and HEOR in Pluralistic Healthcare Systems
Michael F. Drummond, MCom, DPhil, University of York, England, United Kingdom for the ISPOR HTA Council Working Group on HTA in Pluralistic Healthcare Systems*
Background
Much of the discussion of the use of health technology assessment (HTA) in pricing and reimbursement decisions for pharmaceuticals and other health technologies is in the context of healthcare systems with one major payer or HTA agency. We read about the analyses conducted for or by, and decisions made by, IQWiG/G-BA in Germany or NICE in England.1,2 We also read about the similarities and differences of decisions made by the payers/agencies in different countries.3,4 In these (largely) “single-payer” healthcare systems, the conduct and use of HTA is relatively straightforward. The manufacturer submits clinical data (plus an economic model in jurisdictions that require them) to the HTA agency or payer according to the required guidelines, and merely waits for the outcome.
However, if one takes a broad, worldwide view, healthcare systems with one major payer or HTA agency are in the minority. Most healthcare systems are “pluralistic,” with many payers. The most well-known example is the United States, which has a multipayer private healthcare system operating alongside a public system, plus systems serving particular categories of individuals, such as military veterans. The conduct and use of HTA/HEOR in pluralistic healthcare systems is likely to be more complex, since the different payers may have different needs, data requirements and objectives. They may also have budgets of different sizes, with implied differences in willingness to pay for new health technologies. In addition, in pluralistic systems, the resources for conducting HTAs/HEOR are more thinly spread, raising doubts about whether a rigorous assessment can be performed in all cases.
This topic was selected for further study by the ISPOR HTA Council, which established a Working Group to consider these issues and to make recommendations for how the conduct and use of HTA could be improved in pluralistic healthcare systems. The group has recently produced its report and the main findings are summarized here. For more details and an extensive list of references, please consult the full paper.5
Working Group on HTA in Pluralistic Healthcare Systems
The Working Group began by characterizing the types of pluralism observed in healthcare systems worldwide. Private multipayer systems have been mentioned already. These exist most prominently in the United States, but also in some Asian countries. The second type of pluralism, called “parallel healthcare systems,” is most common in Latin American healthcare systems. Here, there is often a public sector for serving disadvantaged populations and for implementing public health interventions, but the major healthcare system is often based on social security financing, based on workers’ and employers’ contributions. Most Latin American countries also have an extensive private healthcare system and some have health services for key groups of workers (eg, government employees, the military). The various mixes of these types of funding vary a lot from country to country. For example, the public sectors in Brazil and Colombia are quite extensive and these countries might be considered close to being “single-payer” systems.
The third type of pluralism is seen in “decentralized healthcare systems,” where the responsibility for the financing and provision of healthcare is devolved by the central or federal government to states, provinces, regions, or territories. Good examples of this approach exist in Canada, India, Italy, and Spain. Although most of the funding is allocated centrally, the main decision-making power concerning the adoption of new health technologies rests with the regions, states, or provinces that frequently have their own HTA bodies.
One of the key insights from this exercise was that several countries exhibited more than one type of pluralism, and that levels of pluralism vary from country to country. Therefore, although it was possible to categorize countries by their main type of pluralism, the real distinctions between countries were much more nuanced. Therefore, the Working Group developed a 3-dimensional taxonomy that could be displayed in a diagram. The figure below, from the main report, categorizes countries in the 3-D pluralism space, according to: (1) the number of payers; (2) the number of parallel healthcare systems and (3) the level of decentralization. The actual judgments the group made about particular countries, which are outlined in detail in the report, could be debated, but the figure nicely illustrates how the nature and level of pluralism varies by country—with those countries with low pluralism being closest to the origin of the figure, those with highest level of pluralism being furthest away. (This ‘honor’ goes to the United States). The other interesting insight from the figure is that the United Kingdom, which we normally think of as a “single-payer” country, does exhibit some pluralism, in that many responsibilities for healthcare are devolved to the 4 nations of the United Kingdom and it also has a small private sector.
Figure. Different types of pluralism across selected countries
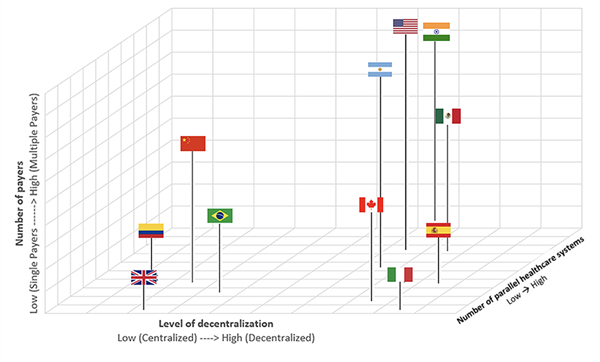
Prior to formulating its recommendations, the group searched for examples of where particular countries had made attempts to deal with the main challenges of conducting and using HTA/HEOR in pluralistic healthcare systems. These examples are too numerous to discuss here, but we mention 2 particularly notable examples. First, there are the activities of the Canadian Agency for Drugs and Technologies in Health (CADTH), which over the years has developed guidelines for the economic evaluation of pharmaceuticals, conducted some demonstration projects in HTA/HEOR and, most importantly, coordinated drug review programs with the participation of provinces and territories. However, CADTH does not have decision-making responsibility, which remains with the provinces and territories. Second, in the United States, in the absence of many federally led efforts in HTA/HEOR, the Academy of Managed Care Pharmacy has developed a format (ie, guideline) for formulary submissions to private health plans and an independently funded body, the Institute for Clinical and Economic Review, has conducted HTAs/HEORs of several new technologies for use by private health plans.6
Recommendations
The group made several recommendations, organized under 5 main themes (See Box).
1. Establishing a national focus for HTA
In single-payer systems, this is achieved by the national government or social insurer establishing an HTA agency. The remits of these bodies vary, but they all provide a focus for HTA efforts in the jurisdiction concerned. The group recognized that in countries with pluralistic healthcare systems there was often a reluctance of the government to get involved in HTA. In some countries, that involvement may not even be welcome! Therefore, the group was agnostic about how a national focus should be established (eg, it could be led by a respected professional society) but felt that such a focus was necessary to promote and to coordinate HTA efforts in all jurisdictions, especially those with pluralistic healthcare systems.
2. Developing a uniform set of HTA methods guidelines
While the nature of pluralism is that different decision makers may have different needs and requirements, in reality the scope for argument about appropriate methods is quite limited. Also, the benefits of giving all those conducting HTAs/HEOR in a given setting clear guidelines far outweigh the benefits of allowing more flexibility in approach. Some methods issues over which there are genuine differences of opinion, such as the inclusion or exclusion of productivity costs and benefits, could be handled in sensitivity analyses.
3. Ensuring that the HTAs are produced in a timely fashion
In single-payer systems, producing timely HTAs is rarely a problem because until the main payer decides to include the new technology for reimbursement, its use will be limited. However, in pluralistic healthcare systems, payers will be making adoption decisions when it suits their needs. Therefore, a new technology may be widely used before the HTA report is available. Historically, this has posed a problem in the United States’ private sector, where some early adopters approve new technologies very quickly, based on their own business considerations, rather than waiting for HTA reports.
This is proving to be one of the major issues in pluralistic systems and the only solutions the group proposed were to (1) start the HTA early (perhaps before licensing approval for the new technology has been given); (2) produce a preliminary assessment, albeit based on limited data, in time to help the early adopters; and (3) revise the HTA as more evidence becomes available.6
4. Facilitating the use of HTA in the local setting
Given that the resources in pluralistic healthcare systems are likely to be thinly spread, it is very unlikely that many payers will have the resources to conduct their own local HTA. Therefore, every possible effort needs to be made to assist the local adaptation and use of HTAs conducted elsewhere. There are a number of possibilities here, such as making interactive models available, training local decision makers in the adaptation and interpretation of HTAs, and developing local cost and epidemiological databases to help populate models with local data.
5. Developing a framework for encouraging transparency in HTA
One of the advantages in single payer countries is that (depending on the country) any HTAs conducted—and the resulting decisions—are made public and shared with any decision makers having an interest. However, in pluralistic systems there are a number of potential barriers to transparency. In multipayer private systems, there may be a reluctance, for commercial reasons, to reveal the details of any analyses that support coverage decisions and the extent to which the decisions are influenced by such analyses. Also, in decentralized and parallel healthcare systems, it may be uncomfortable to reveal that certain new technologies are available in some settings and not others because of different levels of willingness to pay. Inequalities between rich and poor regions of countries, and between different population groups based on insurance coverage, may be inevitable in pluralistic healthcare systems, but are difficult to discuss publicly. The group recognized this but argued that (if possible) the results of HTAs should be made available on a secure website, anonymously if necessary, so at least payers can be aware of what other payers have done and the results obtained.
In making its recommendations, the Working Group acknowledged this was just the start in our understanding of the complexities in the conduct and use of HTA in pluralistic healthcare systems. However, it hoped that relevant jurisdictions may consider the recommendations for adoption, in their own way and in their own time. After all, that’s the nature of pluralism!
*Members of the ISPOR HTA Council Working Group on HTA in Pluralistic Healthcare Systems: Michael Drummond, MCom, DPhil, University of York, United Kingdom; Federico Augustovski, MD, MSc, PhD, Institute for Clinical Effectiveness and Health Policy, Argentina; Devarshi Bhattacharyya, BDS, MPH, MSc, Kalam Institute of Health Technology, India; Jonathan Campbell, PhD, MS, Institute for Clinical and Economic Review, USA ;Nathorn Chaiyakanapruk, PharmD, PhD, University of Utah, USA; Yingyao Chen, PhD, Fudan University, China; Rosa Maria Galind Suarez, BSc, MHEM, Ministry of Health, Mexico; John Guerino, MHS, COEUS Consulting Group, USA; Aurelio Mejia, MSc, Ministry of Trade, Industry and Tourism, Colombia; Michelle Mujoomdar, BSc, PhD, Canadian Agency for Drugs and Technologies in Health, Canada; Daniel Ollendorf, PhD, Tufts Medical Center, USA; Naoko Ronquest, PhD, RTI Health Solutions, USA; Aleksandra Torbica, MSc, PhD, Bocconi University, Italy; Emily Tsiao, PharmD, Premera Blue Cross, USA; John Watkins, PharmD, MPH, BCPS, Premera Blue Cross, USA; Kai Yeung, PharmD PhD, Kaiser Permanente Washington Health Research Centre, USA.
Collaborators: Marcelo Fonseca, University of Saõ Paulo, Brazil; Carly Rodriguez, Moda Health Services, USA
References
1. Isbary G, Staab TR, Volker E, et al. Effect of crossover in oncology clinical trials on evidence levels in early benefit assessment in Germany. Value Health. 2017;21(6):698-706.
2. Chen G, Peirce V, Marsh W. Evaluation of the National Institute for Health and Care Excellence Diagnostics Assessment Program decisions: incremental cost-effectiveness ratio thresholds and decision-modifying factors. Value Health. 2020;23(10):1300-1306.
3. Boucaud-Maitre D, Berdai D, Salvo F. Added therapeutic value of medicinal products for French and German health technology assessment organizations: a systematic comparison. Value Health. 2021;24(3):346-352.
4. Akehurst RL, Abadie E, Renaudin N, Sarkozy F. Variation in health technology assessment and reimbursement processes in Europe. 2017. Value Health. 2021;20(1):67-76.
5. Drummond MF, Augustovski F, Bhattacharyya D, et al. Challenges of health technology assessment in pluralistic healthcare systems: an ISPOR Council Report. Value Health. 2022;25(8):1257-1267.
6. Whittington MD, Pearson SD, Rind DM, Campbell JD. The cost-effectiveness of remdesivir for hospitalized patients with COVID-19. Value Health. 2022;25(5):744-750.

