Long-Term Value Demonstration in Alzheimer’s Disease: Evidence Needs
Paola Barbarino, MA, Chief Executive Officer, Alzheimer’s Disease International, London, England, UK; Anders Gustavsson, PhD, MSc, Partner, Quantify Research; Affiliate, Karolinska Institutet, Stockholm, Sweden; Peter J. Neumann, ScD, Center for the Evaluation of Value and Risk in Health, Tufts University School of Medicine, Boston, MA, USA
The Need for Long-Term Value Demonstration in Alzheimer’s Disease
There is a pressing need for payers, value assessors, and the broader Alzheimer’s community to integrate real-world evidence into long-term value assessments. As discussed in the previous 2 sections of this supplement,1,2 the burden of Alzheimer’s escalates over the course of the disease and extends beyond direct medical costs, affecting caregivers, long-term care systems, economies, and society as a whole. However, current methods of evidence collection—such as clinical trials—do not account for these data elements, nor time horizons. Instead, they tend to focus narrowly on the outcomes that can be measured over a shorter period of time, thus leading to an important evidence gap when considering the longer-term impacts of early interventions.
As we look ahead at the disease-modifying therapies in the pipeline, we can anticipate that future innovative therapies can deliver value over a long period of time; and we can predict that a diversity of stakeholders will benefit from the treatments that delay cognitive, functional, and/or behavioral decline. Long-term value of new treatments must be better understood, and this need grows more urgent as disease-modifying therapies enter phase III trials.
This paper examines the need for long-term evidence as an essential part of Alzheimer’s value assessments. It considers several key topics:
• The need for evidence to support greater access to Alzheimer’s diagnostics earlier in the disease course, which is critical to maximize the value of a potential disease-modifying therapy
• Current gaps in real-world evidence, particularly the need for validated, early-stage outcome measures and the full range of long-term impacts, including for caregivers and healthcare systems
• Focus areas and potential solutions to facilitate long-term value demonstration
• Key actions to build a foundation for long-term value demonstration, as well as innovative payment models to balance affordability, access, and uncertainty
This paper aims to spur both discussion and research on long-term outcome measurement and value assessment in Alzheimer’s disease. It rests on the foundational premise that value assessments must adopt a broad perspective that considers a treatment’s real-world benefits over time in order to measure the potential value of a novel Alzheimer’s therapy.
Evidence Needs for Early Detection and Diagnosis
Evidence needs in Alzheimer’s disease start with detection and diagnosis. Access to screening, detection, and diagnosis is critical to effectively address the disease, yet current policies often do not support access to the necessary tests and tools because of a perceived lack of evidence of benefit. Greater evidence on the value of detection and diagnosis, including real-world data collected over an extended period of time, can help to make the case for access, laying the groundwork for earlier, more effective treatment with a potential disease-modifying therapy.
"Long-term value of new treatments must be better understood, and this need grows more urgent as disease-modifying therapies enter phase III trials."
This is critically important, given the growing consensus that to detect and treat Alzheimer’s disease in its earliest stages, health systems must have a strong emphasis on timely diagnosis. This would enable a disease-modifying treatment to have the greatest potential to alter or slow disease progression, maximizing the benefits for patients and potentially delaying care interventions. It should also reduce overall costs to health and care systems, families, and society.3 Crucially, detecting Alzheimer’s disease early allows families and people living with Alzheimer’s disease to prepare adequately for what is to come. However, the benefits of this approach accrue over time and entail a broader set of outcomes than are currently measured. This creates the need for earlier, better real-world evidence for both diagnosis and treatment.
Figure 1 illustrates the potential benefits of this approach. As shown, an effective disease-modifying therapy would delay the decline in cognition and function, thereby giving patients more time in better health, reducing total costs and burdens by delaying the need for formal care, and potentially mitigating indirect costs like lost productivity and caregiver impacts.
Figure 1: Long-term trajectory of people with Alzheimer’s disease and the potential effect of a disease-modifying treatment.

Improving access to screening, detection, and diagnosis is the first step. Currently, it is estimated that at least half of patients with Alzheimer’s disease and dementia are undiagnosed, and diagnosis, when it occurs, often happens 2 to 5 years after the onset of symptoms.4 Therefore, diagnosis rates must be improved to maximize the benefits of a disease-modifying therapy.
However, certain coverage recommendations and decisions for detection and diagnostics currently cite a need for more evidence of value. For example, in the United States, the US Preventive Services Task Force does not currently recommend cognitive impairment screening for older adults.3 Further, the US Centers for Medicare and Medicaid Services has ruled that there is insufficient evidence to justify broad coverage of neuroimaging diagnostics like positron emission tomography (PET) and magnetic resonance imaging.5
"Gaps in evidence for diagnostics demonstrate the broader challenge of a lack of real-world data in Alzheimer’s disease."
Real-world evidence can help to show the value of early detection, and if the evidence warrants it, to expand access to diagnostics, especially as disease-modifying therapies approach the market. For example, the Alzheimer’s Association led the Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) study to investigate the benefits of amyloid PET diagnostics with real-world evidence.5 Enrolling over 18,000 Medicare beneficiaries, the IDEAS study found that amyloid PET led to changes in patient management in 60% of patients with mild cognitive impairment and 64% of patients with dementia of uncertain cause.6 This imaging data resulted in a different diagnosis 36% of the time.6 Furthermore, these benefits were found without a disease-modifying therapy available. If such a therapy were to become available, the potential real-world benefits of diagnosis would be even greater.
This work illustrates how real-world evidence can build the case for greater access to diagnosis. While it focuses specifically on PET testing, similar dynamics could apply to other procedures for early detection, such as cognitive screening, cerebrospinal fluid testing, or, eventually, blood-based biomarkers. Similar efforts will potentially be needed to examine the long-term, real-world benefits of other approaches to early detection and diagnosis.
Data Gaps: Assessing Needs in Real-World Evidence
Gaps in evidence for diagnostics demonstrate the broader challenge of a lack of real-world data in Alzheimer’s disease. Currently, most data on Alzheimer’s disease come from clinical trials that evaluate drug safety and efficacy with strictly controlled protocols for a set period of time.7 This approach is unlikely to capture the full benefits of a disease-modifying therapy, which may include more outcomes and a longer period of time than measured in clinical trials.
For example, in the United Kingdom, the National Institute for Health and Care Excellence’s guidelines state that the “time horizon for estimating clinical and cost-effectiveness should be sufficiently long [emphasis added] to reflect all important differences in costs or outcomes between the technologies being compared.”8 A lifetime perspective is appropriate in most cases and data should be extrapolated beyond the duration of a clinical trial.9
But what outcomes should be measured directly versus extrapolated, in which populations, and for how long?
To answer these questions, the European ROADMAP project (Real-World Outcomes Across the Alzheimer’s Disease Spectrum: A Multimodal Data Access Platform) “aim[ed] to deliver a series of methods and tools that will allow the scalable, transferable integration of data on patient outcomes in the real world.”10 As a starting point, ROADMAP assessed current data sources and identified existing gaps in real-world evidence.
This approach is illustrated in Figure 2, which shows the ROADMAP project’s “data cube.”7 The cube’s 3 axes represent data sources, disease stages, and outcomes, showing where additional research is needed. Although ROADMAP assessed diverse sets of data, including population-based databases, national registries, electronic health records, disease registries, and randomized controlled trial data, the cube makes clear that no data source is comprehensive. Even valuable sources are limited in the outcomes and data sources they measure. As a visual representation of these limits, the cube aids researchers in targeting gaps early, enabling them to be filled in advance of any therapy’s review by a regulatory or value assessment body.
Figure 2: The ROADMAP Data Cube visualizes the project’s 3 key activities and how they contributed to identify key gaps across Alzheimer’s disease stages, outcomes, and data sources. Colors indicate the relative availability of relevant data. Darker colors show where more relevant data exist, while lighter colors indicate where data needs are greater.
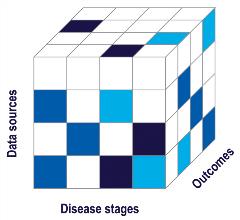
ROADMAP has identified 2 of the most pressing real-world evidence needs: (1) validated outcome measures for the earliest asymptomatic stages of Alzheimer’s disease; and (2) real-world evidence that shows a therapy’s long-term effects across numerous stages of disease, including on outcomes, caregivers, and healthcare systems.7
First, validated and well-established outcome measures are necessary in early symptomatic stages of Alzheimer’s disease (including prodromal Alzheimer’s), as new treatments will likely target these stages of the disease for the first time. Since a treatment that is effective in these stages will need to prevent or delay clinical symptoms, the research community must build consensus on what constitutes a meaningful delay in disease progression. However, information about early clinical symptoms of Alzheimer’s is limited, impeding accurate measurement of the early effects of the disease.7 To fill this evidence gap, the research community can identify effective and reliable instruments to measure cognition as subtle symptoms start to emerge. These instruments must be applicable in real-world environments like the home—not just in strictly controlled clinical trials.
"From the patient perspective, there is a lack of granularity in the knowledge about the different patient subgroups and disease substages, especially in the early stages of the disease."
Although there are many validated screening tools used to detect mild cognitive impairment (MCI), most are only validated in the memory clinic setting, rather than in the general population.11 There are currently 80 pen-and-paper tests for MCI that have been validated in memory clinic settings, and there exist validation studies for 7 computer-based MCI screening tests.11 However, only 2 pen-and-pencil tests for MCI detection have been validated in a population-based cohort, and only 1 computer-based test has been validated in a population-based cohort.11 Although these tests reflect meaningful outcomes to patients, a lack of validated real-world data is a barrier to consensus on how to interpret clinically meaningful cognitive changes.
Second, real-world evidence is needed to assess a therapy’s long-term impacts across a broader set of outcomes than those typically used in clinical trials to support product registration, including outcomes of importance for caregivers and healthcare systems. As discussed in the first paper of this supplement by Garrison et al,1 these benefits constitute a significant share of potential value in Alzheimer’s disease.
In particular, ROADMAP proposed a framework for using real-world evidence to assess caregiver impacts.7 The framework holds that caregiver-relevant outcomes should be established by consensus. These outcomes may include quality of life, health status, loss of income, and caregiver time; all of which are key factors in health economics modeling. Overall, ROADMAP called for international consensus on which outcomes will inform regulatory and health technology assessment decisions. With consensus, international coordination is needed to actually generate the real-world evidence on these outcomes. Importantly, pharmacoeconomic analysis needs data that will enable it to consider distinctions between national and regional settings, such as differences in relevant outcomes, costs, and unique care delivery characteristics.
By filling these real-world evidence gaps, stakeholders can provide a more accurate analysis of the true costs of Alzheimer’s disease across an entire society. This would establish the basis for value assessment that more fully captures the disease’s immense costs and the potential value of treatment advances.
Potential Solutions: Identifying Focus Areas for Long-Term Value Demonstration
To address these gaps, progress is needed on 2 fronts: (1) data collection; and (2) leveraging the data in value assessments.
Data Collection
In the area of data collection, there is a critical need for more evidence on financial impacts and total costs. Real-world evidence is often used to assess whether results from a clinical trial carry over into the real world. But it can also be used to address one of the most important gaps in current Alzheimer’s value assessment: the overwhelming financial strain on families, communities, the workforce, and health systems. These costs extend far beyond the direct expenses of medical treatment and care, but the potential value of reducing these real-world impacts is neither measured by clinical trials nor fully captured by existing value assessment frameworks.
"Building the consensus, infrastructure, and evidence base for long-term value demonstration in Alzheimer’s disease will require concerted efforts and collaboration between many different stakeholders over the course of multiple years."
To better evaluate a new disease-modifying therapy’s effects on the total costs of Alzheimer’s disease, real-world evidence should expand the scope of cost-effectiveness evaluations beyond the healthcare payer perspective. The financial cost of Alzheimer’s disease is distributed throughout society, with patients and caregivers bearing the brunt of the economic burden. In 2016, the estimated global costs of Alzheimer’s disease and dementia were $948 billion, and costs are projected to increase 15.94% each year as disease prevalence and care expenses rise.12,13 Critically, direct medical costs—those covered by healthcare payers—account for just 16% of total costs, while social care costs account for 42.3%, and informal care and indirect costs account for 41.7%.14
A new disease-modifying therapy in Alzheimer’s disease can potentially lower not just direct medical costs, but the much larger downstream nonmedical, indirect, and spillover costs. These savings compound over time and fall outside the balance sheet of healthcare payers.
Real-world evidence can help correct these evaluations, but there are challenges. Overall, there is a high degree of heterogeneity in the Alzheimer’s disease community. From the patient perspective, there is a lack of granularity in the knowledge about the different patient subgroups and disease substages, especially in the early stages of the disease. From the provider perspective, there is a lack of consensus on the right outcomes to include in real-world evidence collection. Further, data and biomarkers are rarely digitalized nor harmonized between different systems, and there are often technical and legal barriers to data infrastructures.
Initiatives are attempting to solve these challenges. For example, the US National Alzheimer’s Coordinating Center (NACC) offers rich data on thousands of patients that can be accessed by researchers all over the world.15 The Swedish dementia register (Svedem), another example, has complete national coverage of specialist care and approximately 75% of primary care centers, collecting longitudinal clinical data on some 90,000 dementia patients to-date.16,17 These data sources have been and can be used to develop models for assessing the long-term benefits of new candidate treatments.17,18
However, there is still room for improvement, particularly in 3 interlinked areas: the Alzheimer’s model, outcomes, and data. First, the model representation of Alzheimer’s disease can be improved. As noted above, it should be comprehensive enough to encompass the impact on all stakeholders, and therefore have a societal and long-term perspective. It should also be granular enough to represent the continuous and slowly progressive disease which is Alzheimer’s. Today, we have a rather granular representation of the dementia stage, whereas the predementia stage is often crudely represented as an annual conversion from MCI to dementia. Finally, we can better acknowledge the heterogeneity of the Alzheimer’s population, by considering the impact on different subgroups (eg, as defined by age or genetic profiles) and making sure all cohorts under evaluation are well defined with stringent eligibility criteria.
Second, outcomes are particularly difficult to assess in Alzheimer’s disease because there are no clear, clinically meaningful events, such as a fracture or stroke in other diseases. Instead, complex measurements of pathology biomarkers and clinical symptoms are collected within trials, but with limited meaningfulness to patients.19 Real-world data can help describe how these intermediate outcomes are connected to the longer-term outcomes of value to patients, caregivers, and society as a whole. Also, digitalization of the measurement of clinical symptoms may improve their accuracy and efficiency in both clinical practice and research.20,21
Third, data have inherent challenges that have the potential to be solved by effort and collaboration. An effective infrastructure is needed to compile the large and longitudinal databases that are required to describe this disease. There are technical and legal barriers, which stem from the sensitive nature of health data. These challenges can be overcome by engaging all stakeholders, including policy makers and patient organizations, conveying a joint message that these data are essential for improving public health.
Collaboration is also needed to harmonize the data, such as establishing uniform data and core outcomes sets. This would enable comparison and pooling of multiple data sources. Some types of data will not be representative across settings and country borders, which re-emphasizes the need for well-defined study populations.
Finally, validation efforts should be made to compare and explain remaining differences across different data sources. As an example, the International Pharmaco-Economic Collaboration on Alzheimer’s Disease (IPECAD), held a workshop in September 2020 where a dozen developers ran their Alzheimer’s models based on a variety of the available real-world data sources, but with a jointly agreed treatment scenario. The inputs and outcomes were compared and scrutinized in an attempt to systematically explain differences across models, and to learn from this process (see IPECAD.org).
Integrating Data Into Value Assessment
At the same time as data collection improves, value assessors can capture the costs of Alzheimer’s across society during a longer time span. Cost-effectiveness frameworks can reflect the many stakeholders who benefit from new treatments. Frameworks can include outcomes like quality of life, needs for full-time care, levels of dependency, and the onset of advanced disease states.
Furthermore, value assessors can use sensitivity analyses to test alternative discounting frameworks. Discounting calculates the current value of financial benefits that will be gained in the future, enabling measurement of changing therapy benefits over time. However, current discounting methods, which follow country-specific guidelines, struggle to make accurate assumptions about the magnitude and timing of drug effects.22 As a result, they can overvalue short-term benefits and undervalue medium- and long-term benefits.22 As the greatest financial benefits of a new disease-modifying therapy will come in the medium- to long-term, alternative discounting methods are needed to accurately value new Alzheimer’s drugs.22
Building the consensus, infrastructure, and evidence base for long-term value demonstration in Alzheimer’s disease will require concerted efforts and collaboration between many different stakeholders over the course of multiple years. It is important to accelerate progress in this area in order for real-world evidence to inform value decisions.
Next Steps: Building a Foundation of Long-Term Value Demonstration and the Potential Role of Innovative Payment Models
Progress is needed on 2 fronts: (1) creating the foundation for long-term value demonstration; and (2) exploring the role of innovative assessment and payment models.
In the near-term, stakeholders can use models to identify key drivers of long-term value, especially by starting models earlier in a patient’s life course and modeling real-world scenarios, considering factors like diagnosis, subgroups, and adherence. Stakeholders must also start to fill data gaps, like those mentioned above, as well as costs and utilities by stage, comorbidities, caregiver utilities, and societal impacts. Stakeholders can also validate biomarkers and consider health and societal perspectives.
However, while a larger base of real-world evidence is essential, it will not solve all Alzheimer’s value and payment challenges, especially if a new therapy is approved before these efforts are completed. Therefore, there is also a need for innovative payment models, which can balance access, the potentially high cost of a new treatment, and the uncertainty of long-term outcomes. Two potential approaches are outcomes-based payment models and subscription agreements. Outcomes-based payment models leverage real-world evidence to substantiate value, which affects price and access. Subscription models are agnostic of product performance and leverage innovative payment cycles.
"To further facilitate drug access, governments must ensure positive long-term incentives for investing in drug development."
Within outcomes-based payment models, a performance-warranty approach compensates drug manufacturers based on patient outcomes obtained with real-world use of the product.3 If a treatment is observed to be effective based on predetermined criteria, the manufacturer receives full compensation. If it does not reach efficacy requirements, the manufacturer receives only partial compensation. This approach distributes risk between manufacturers and payers, while providing access to patients. In effect, it reduces uncertainty in the treatment outcomes by “building in” real-world evidence for payment.
Costs can also be distributed with subscription payment models. In this approach, a health system pays manufacturers a fixed amount for drug access, regardless of the number of patients served.3 This arrangement allows for cost management as patient populations increase, while offering a reliable revenue stream for manufacturers.
As one example, the subscription model has been successfully applied to hepatitis C. When hepatitis C therapies were introduced in 2015, they cost $100,000 per patient.3 To manage costs while serving a large patient population, the states of Washington and Louisiana negotiated subscription agreements. These arrangements offer a model that can be adapted to Alzheimer’s disease because they benefit payers, patients, and drug manufacturers alike.
To further facilitate drug access, governments must ensure positive long-term incentives for investing in drug development. At the same time, drug manufacturers must clearly demonstrate a therapy’s value with sound empirical evidence.3 Innovative payment models can help to balance these 2 imperatives, while ensuring that evidence barriers do not impede access for patients.
Preparing for the Future of Long-Term Alzheimer’s Value Assessment
Although long-term evidence needs in Alzheimer’s are vast and will certainly evolve as research progresses, leading scholars point to several key conclusions:
• A safe and effective disease-modifying Alzheimer’s treatment will likely deliver value over the course of many years and many different stakeholders, creating a need for long-term value demonstration.
• Real-world evidence on diagnostics can help to enable widespread early detection, which is critical to maximize the benefits of disease-modifying treatment.
• Gaps in real-world evidence impede long-term measurement of disease cost to society and potential value.
• Greater collaboration is needed to facilitate real-world evidence and long-term value demonstration, including consensus on outcomes, data, and modeling.
• Innovative payment models can help to ensure a therapy’s affordability and access.
We urge further research to develop real-world evidence initiatives and innovative value frameworks that can accurately, holistically, and equitably assess the value of future therapies in Alzheimer’s disease. •
References
1. Garrison LP Jr, Baumgart M, El-Hayek YH, Holzapfel D, Leibman C. Defining elements of value in Alzheimer’s disease. Value & Outcomes Spotlight.2021;7(1S):S7-S11.
2. Basu A, Lynn N, Peschin S, Resendez J. Value assessment in Alzheimer’s disease: a focus on equity. Value & Outcomes Spotlight. 2021;7(1S):S12-S17.
3. Lin PJ, Cohen JT, Neumann PJ. Preparing the health-care system to pay for new Alzheimer’s drugs. Alzheimers Dement. 2020;16(11):1568-1570. https://doi.org/10.1002/alz.12155
4. Fowler NR, Head KJ, Perkins AJ, et al. Examining the benefits and harms of Alzheimer’s disease screening for family members of older adults: study protocol for a randomized controlled trial. Trials. 202019;21(1):202. https://doi.org/10.1186/s13063-019-4029-5. PMID: 32075686; PMCID: PMC7031904.
5. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286-1294. https://doi.org/10.1001/jama.2019.2000
6. Original IDEAS Study. New IDEAS. https://www.ideas-study.org/Original-Study. Accessed December 1, 2020.
7. Bouvy JC, Jonsson P, O’Rourke D, et al. Regulatory and health technology assessment considerations for disease-modifying drugs in Alzheimer’s disease. CNS Drugs. 2018;32(12):1085-1090. https://doi.org/10.1007/s40263-018-0581-x
8. The Reference Case: Guide to the Methods of Technology Appraisal 2013: Guidance. The National Institute for Health and Care Excellence. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case. Published April 4, 2013. Accessed December 2, 2020.
9. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093-1103. https://doi.org/10.1001/jama.2016.12195
10. ROADMAP: Real world outcomes across the AD spectrum for better care: multi-modal data access platform. IMI Innovative Medicines Initiative. https://www.imi.europa.eu/projects-results/project-factsheets/roadmap. Published January 11, 2016. Accessed December 1, 2020.
11. De Roeck EE, De Deyn PP, Dierckx E, Engelborghs S. Brief cognitive screening instruments for early detection of Alzheimer’s disease: a systematic review. Alzheimers Res Ther. 2019;11(1):21. https://doi.org/10.1186/s13195-019-0474-3
12. Xu J, Zhang Y, Qiu C, et al. Global and regional economic costs of dementia: a systematic review. Lancet. 2017;390:S47. https://doi.org/10.1016/S0140-6736(17)33185-9.
13. Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1-7. https://doi.org/10.1016/j.jalz.2016.07.150
14. Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15(5):455-532. https://doi.org/10.1016/S1474-4422(16)00062-4
15. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249-258. https://doi.org/10.1097/WAD.0b013e318142774e
16. Haaksma ML, Eriksdotter M, Rizzuto D, et al. Survival time tool to guide care planning in people with dementia. Neurology. 2020;94(5):e538-e548. https://doi.org/10.1212/WNL.0000000000008745
17. Wimo A, Handels R, Winblad B, et al. Quantifying and describing the natural history and costs of Alzheimer’s disease and effects of hypothetical interventions. J Alzheimers Dis. 2020;75(3):891-902. https://doi.org/10.3233/JAD-191055
18. Green C, Handels R, Gustavsson A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: an open-source modeling framework. Alzheimers Dement. 2019;15(10):1309-1321. https://doi.org/10.1016/j.jalz.2019.05.004
19. Tochel C, Smith M, Baldwin H, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Dement (Amst). 2019;11:231-247. Published March 7, 2019. https://doi.org/10.1016/j.dadm.2018.12.003
20. Dorsey ER, Papapetropoulos S, Xiong M, Kieburtz K. The first frontier: digital biomarkers for neurodegenerative disorders. Digit Biomark. 2017;1(1):6-13. https://doi.org/10.1159/000477383
21. Wild K, Howieson D, Webbe F, Seelye A, Kaye J. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement. 2008;4(6):428-437. https://doi.org/10.1016/j.jalz.2008.07.003
22. Attema AE, Brouwer WBF, Claxton K. Discounting in economic evaluations. Pharmacoeconomics. 2018;36(7):745-758. https://doi.org/10.1007/s40273-018-0672-z

