Improving the Acceptability of RWE: What's New in 2022?
Tyler D. Wagner, PharmD, Virginia Commonwealth University School of Pharmacy, Richmond, VA, USA; Jacinda Tran, PharmD, MBA, Comparative Health Outcomes, Policy, and Economics (CHOICE) Institute, University of Washington, Seattle, WA, USA
To kick off the real-world evidence (RWE) subprogram at ISPOR 2022, experts from the US Food and Drug Administration (FDA), the European Medicine Agency (EMA), and Harvard Medical School aimed to better communicate new trends and uses of real-world data (RWD) in 2022 and beyond.
FDA guidance on RWD and RWE
In 2016, the 21st Century Cures Act (Cures Act) was signed into law to help accelerate medical product development and to bring new innovations and advances to patients who need them in a more quick and efficient manner. The Cures Act also allowed the FDA to establish a program to modernize clinical trial designs and evaluate the potential use of RWE. More than 6 years after the passage of the Cures Act, the terms RWD and RWE are being used inconsistently and interchangeably, and according to John Concato, MD, MPH (US Food and Drug Administration, USA), it’s hampering progress in the field. While RWD are routinely collected and contain information about patient care or healthcare delivery (eg, electronic health records [EHR], medical claims, product and disease registries, wearables), RWE is the clinical evidence generated from observational studies, externally controlled trials, or randomized trials (eg, pragmatic trials).
In a 2022 study by Concato and colleagues, the authors highlighted the 2 most common misconceptions regarding these terms. The first misconception is that RWE and RWD are new concepts. “The sources of data and types of study designs haven’t fundamentally changed, but electronic access to more detailed clinical data is evolving and the data are becoming more relevant and reliable,” note the authors. The second misconception is that a simple dichotomy of randomized trials versus observational studies exists. In reality, clinical trials are defined by assignment of treatment according to protocol, and single-arm trials face challenges similar to those in observational studies in determining whether differences in clinical outcomes represent actual treatment effects,” said Concato.
Figure 1, found in Concato’s NEJM publication, depicts the use and increasing reliance of RWD across various study designs and the resulting generation of RWE. To increase the acceptance of RWE in industry, the FDA has drafted guidance that includes 4 drafts that address RWD within EHRs and medical claims data, RWD within registries, data standards for drug submissions that contain RWD, and considerations for use of RWD and RWE. Concato discussed Prograf’s new indication in lung transplantation as a recent example of FDA approval based on RWE.
Figure 1. Reliance on RWD in representative types of study designs.
Source: Concato J, Corrigan-Curay J. Real-world evidence—where are we now? N Engl J Med. 2022;386:1680-1682.
HARPER: A Joint ISPE/ISPOR Task Force Initiative
Shirley Wang, PhD (Harvard Medical School, USA) discussed the HARmonized Protocol Template to Enhance Reproducibility (HARPER) hypothesis for evaluating RWE studies on treatment effects—a protocol that stemmed from an ISPE/ISPOR joint task force. Wang highlights how the template is “a tool for unambiguous communication about study implementation” that aims to assess the ambiguity that can limit the utility of database studies in healthcare decision making.
Through further discussion of HARPER, innovative sections of the template are highlighted, such as how the template provides an amendment section that is continually updated and includes free text on context and rationale for scientific choices as well as tables and figures to summarize the operational definitions. The most comprehensive section is on research methods, where the task force elaborates on the context and rationale for the study design and the use of a design diagram to communicate how the cohort is created (similar to a visual abstract). When asked about next steps for HARPER, Wang shared that “we would like to try and test this a little bit more with engagement of people through HTA and FDA assessment to see if we can gain more learnings and insights, as well as trying to integrate with study registries”.
The joint task force agreed that the design diagram should be a core component of the HARPER template. In a study by Happe et al, the authors found that only 42% of database studies in JMCP included a design diagram, even though they are strongly recommended. Stay tuned to Value in Health for the upcoming publication the joint ISPE/ISPOR task force on the HARPER study!
Methodological Working Party and DARWIN-EU
Xavier Kurz, MD, PhD (European Medicines Agency, The Netherlands) discussed the role of RWD and RWE in applications for marketing authorization and extension of indication. He highlighted that for optimal use of RWE, it is important to interact with regulators at the early stages, assess the needs of all stakeholders, and enable tracking of RWE use.
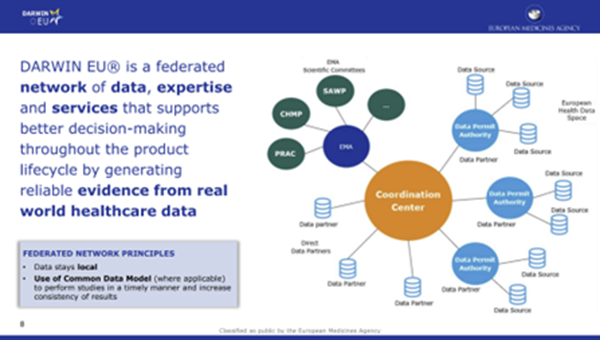
Kurz further elaborated on the Methodological Working Party (new in 2022) that gathers experts on the assessment of RWE to discuss methodological topics relevant to EMA committees. Another group, the Data Analysis and Real-World Interrogation Network (DARWIN EU), a federal network of data, expertise, and services (Figure 2), supports better decision making throughout the product life cycle by generating reliable evidence from RWD. Figure 2 depicts the components of DARWIN EU, which consists of a coordination center that interacts with the EMA, data permit authorities, and data partners.
Figure 2. DARWIN EU network of data
For more coverage on the RWE Subprogram at ISPOR Annual 2022, check out the following link. RWE studies can be registered at: www.ispor.org/RWEregistry or clinicaltrials.gov.


