Patient- and Caregiver-Perceived Challenges to Effective Participation in Health Technology Assessment Activities in Europe
Priyadharshini Ramakrishnan, MDS, Julia Poritz, PhD, Jordan Godwin, MA, and Elizabeth Hubscher, PhD, Cytel Inc, Waltham, MA, USA
Introduction
Encouraging patient engagement (PE) in the health technology assessment (HTA) process strengthens the equity, relevance, accountability, and credibility of decision making.1 Patients and patient representatives are an important source of information, as their insights into the lived experience of disease improve our understanding of unmet needs and the impact of the disease/therapy in question. Many novel technologies lack tangible economic benefits but provide promising clinical outcomes.2 These situations require that the lived experience of patients is captured to emphasize the potential benefit of emerging treatments and their potential value to those stakeholders most directly affected by their recommendation for approval—the patients.2 Although the European Network for HTA (EUnetHTA) recognizes that patient perspectives are “an essential part of the evidence base that is integral to the interdisciplinary process of an HTA,”3 there is significant variability in PE among HTA bodies in Europe.4 As treatments evolve and clinical outcomes become more complex, incorporating patient perspectives and experiences into the HTA process can aid in the development of more informed recommendations.2 Our objective was to identify the challenges perceived by patients and their representatives for effective participation in HTA activities in Europe and to review the existing guidelines published by HTA organizations from the European Union (EU4) and the United Kingdom (UK) that address these challenges.
"As treatments evolve and clinical outcomes become more complex, incorporating patient perspectives and experiences into the HTA process can aid in the development of more informed recommendations."
Our approach
We conducted a targeted literature review in PubMed to identify studies published between January 2012 and April 2022 describing the challenges reported by patients, caregivers, and patient representatives for active participation in HTA activities in Europe. Search terms included ”patient engagement,“ “health technology assessment,“ ”challenges,“ ”barriers,“ ”patient participation,“ “patient involvement,” “stakeholder expectations,” “patient perspective,” “patient organization,” “patient representative,” “caregiver,” “carer,” “public involvement,” and “patient preferences.” Observational studies were included, whereas narrative and systematic reviews, non-English studies, and studies with participants from non-European countries were excluded. We also reviewed recently available guidance from the National Institute for Health and Care Excellence (NICE; UK), Haute Autorité de Santé (HAS; France), Institute for Quality and Efficiency in Health Care (IQWiG; Germany), Agenzia Italiana del Farmaco (AIFA; Italy), and Agencia Española de Medicamentos y Productos Sanitarios (AEMPS; Spain) that address these challenges to PE in HTA.
What do patients and patient representatives perceive as challenges?
The targeted literature review identified 12 cross-sectional studies involving patients, caregivers, and representatives from patient organizations across Europe.4-15 This included participants from the United Kingdom (n=5), The Netherlands (n=4), France (n=3), Germany (n=3), Italy (n=3), Ireland (n=2), Romania (n=2), Spain (n=2), Sweden (n=2), Switzerland (n=1), Belgium (n=1), Denmark (n=1), and Finland (n=1). Most of the studies involved patient organizations (n=10); patients and caregivers were involved in 3 studies, one of which also included patient organizations.4-15 The identified studies employed various methods including questionnaires (n=7), interviews (n=4), consultations (n=3), workshops (n=2), and seminar and focus group discussions (n=1), as well as a combination of these to identify challenges to HTA involvement experienced by patients and patient representatives.4-15
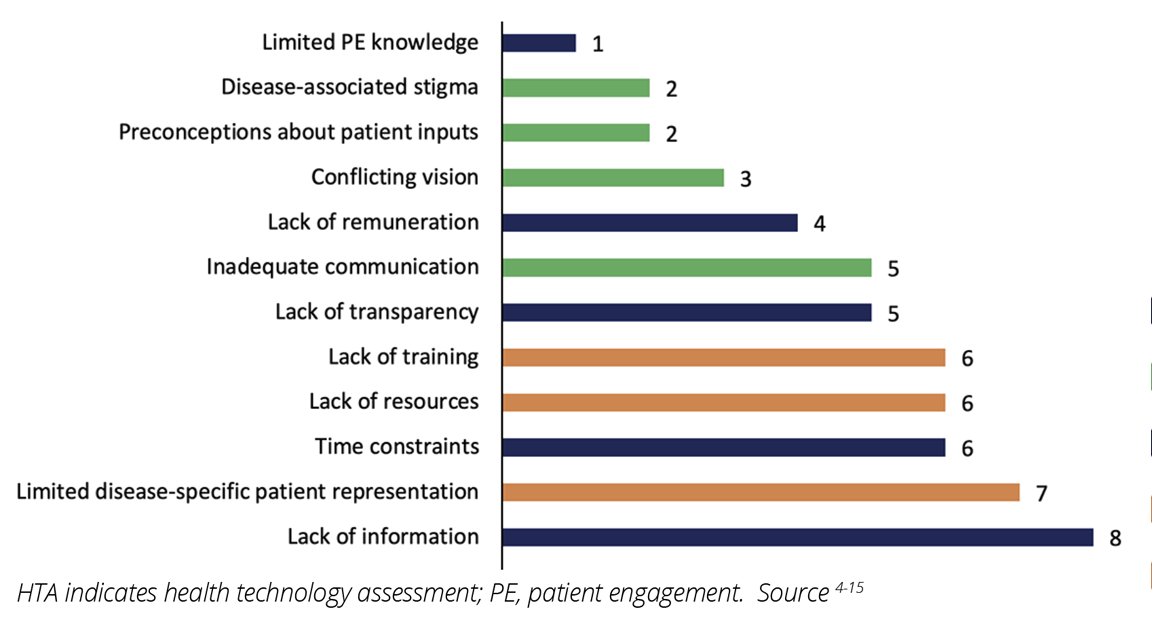
Across the 12 studies, 13 unique challenges were identified, including political, economic, sociocultural, methodological, and technological challenges.4-15 Most of the challenges experienced by patients and patient representatives were associated with the HTA process and the bilateral relationship between HTA agencies and the patients or patient organizations (Figure 1). Challenges for participation in HTA also occurred at the patient or patient representative level, such as limited disease-specific patient representation in patient organizations, (n=7), as well as lack of adequate training and resources for participation in HTA (n=6 each) (Figures 1 and 2).4-15 The lack of information from HTA agencies about the HTA process (eg, details about the technology being assessed and how reimbursement decisions are made) and HTA agencies overlooking the value of patient inputs (n=8, each) were the most commonly perceived barriers to effective PE in HTA (Figure 2).4,5,8-13,15 Specifically, in some cases, patient organizations perceived their involvement in the HTA process as “superficial” and “tokenistic.”5,10 Limited PE knowledge among HTA agencies was the least reported challenge (n=1).5
Figure 1: Studies reporting challenges for effective patient engagement in HTA activities
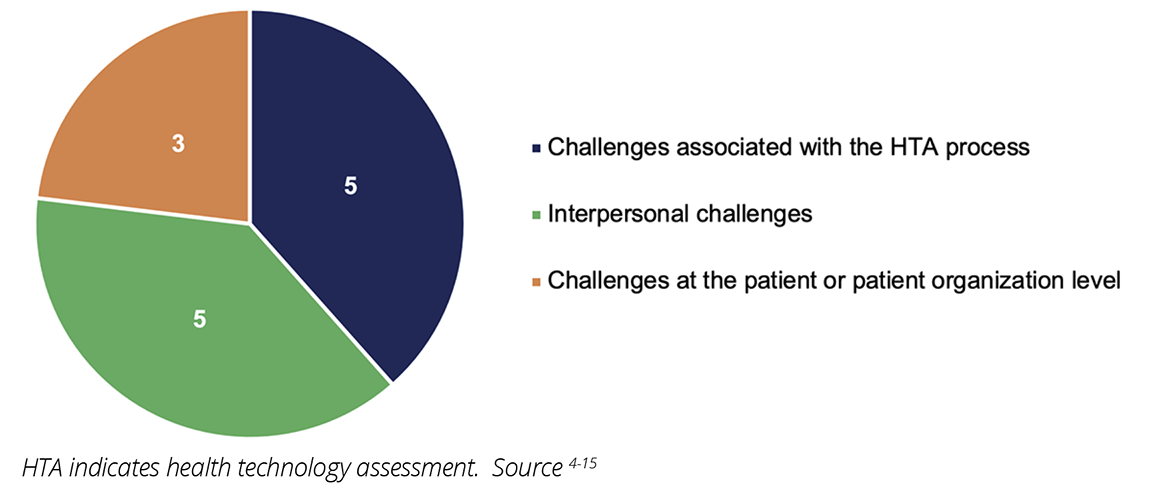
Figure 2: Challenges reported by patients and/or patient organizations for participation in HTA activities in Europe
Do existing guidelines from HTA bodies address these challenges?
Of all the HTA bodies assessed in this search, NICE had the clearest guideline for how patients can and are expected to participate in the HTA process (Table 1).16 The NICE guideline specifies the patient expert nomination and selection process at the outset and details patient expert participation in committee meetings. Patients and patient representatives are involved early in the HTA process from the scoping stage in outlining the methods to the appraisal of technology assessment and scientific advice stage.16 The guideline distinctly outlines the role of patients and their representatives including their participation in workshops and committee meetings and provision of comments on recommendations. NICE also recognizes that both strategy and support for including patients in the HTA process are necessary and provides proactive outreach, training, and mentoring by engaging a dedicated patient expert liaison and documenting the impact of the patient experts’ contributions.16
Table 1: Guidelines addressing challenges for PE in the HTA process in Europe
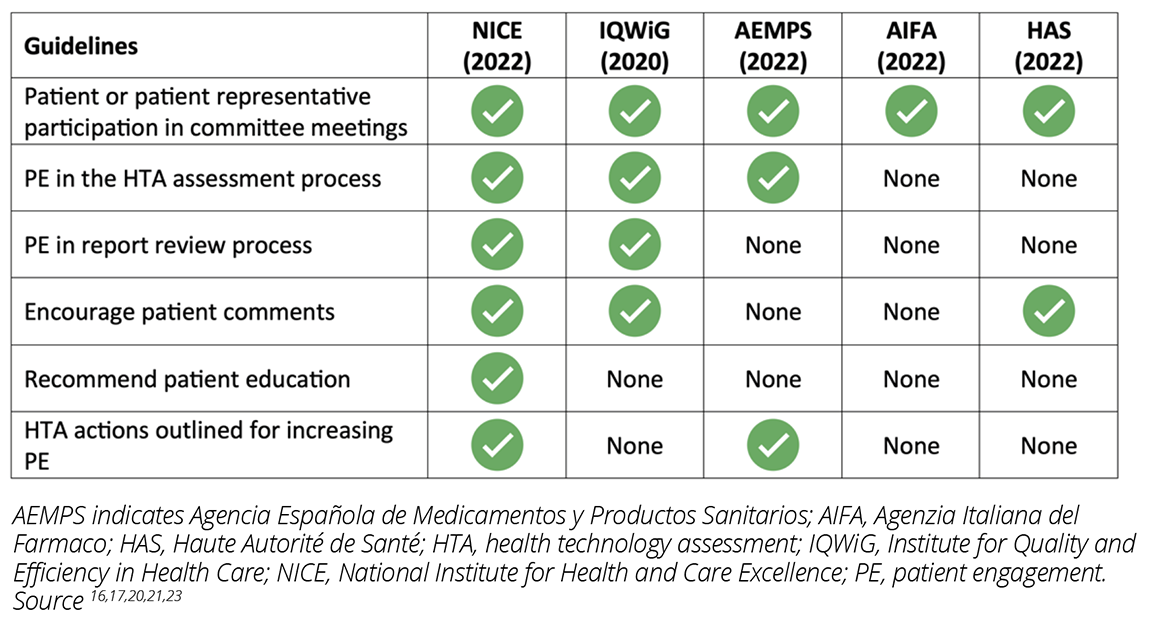
IQWiG recognizes the importance of patients’ and caregivers’ perspectives by involving them in the selection of topics for HTA and integrating their perspectives in defining patient-relevant outcomes and scientific assessment.17 IQWiG welcomes stakeholder comments and considers these comments in its HTA. During dossier assessments and economic evaluations of technology, IQWiG invites comments from patients and patient organizations through formal questionnaire assessments. Patients and their representatives are also members of the Board of Trustees and have the opportunity to review the reports before user testing.17
Patient participation in the HTA process in Spain is limited to representation in the Governing Council on technical committees such as the Committee for Medicines for Human Use and the Committee for Medical Devices.18 However, the Spanish Network of Agencies for Assessing Health Technologies (RedETS), which is responsible for the assessment of technologies for inclusion in the Spanish Common Benefit Portfolio, published a methodological guideline for patient involvement in HTA.19 The guideline encourages the contribution of patients or patient representatives in the protocol and preliminary report review process and in the assessment for including patient-based evidence.19
"The gaps in the existing guidelines reveal that the burden is on HTA bodies to ensure that patients and
patient representatives are included and have access to the requisite resources for effective participation in the HTA process."
In France, PE in HTA is still in the early stages and is limited to proposing topics for assessment to HAS.20 Similarly, despite PE being in its infancy in Italy (ie, restricted to participation in Open AIFA meetings),21 AIFA is involved in enabling systemic and meaningful PE in HTA through its participation in the Patients Active in Research and Dialogues for an Improved Generation of Medicines project (PARADIGM), a European partnership aimed at enabling PE.22
In 2018, the European Patients’ Academy on Therapeutic Innovation (EUPATI) published specific recommendations for patient involvement in HTA. The guideline recommended that HTA bodies across Europe engage in outreach and educational activities, allow wider patient involvement, and provide compensation for patient involvement.1 Although these recommendations were made 5 years ago, our study shows that methodological challenges still exist for patients and patient representatives, highlighting the need for improvement.11 Although most of the guidelines identified in the present study were published after the 2018 EUPATI recommendations, the review of guidance documents showed that for the most part, PE in HTA is limited to proposing topics for assessment or commenting via questionnaires when PE could be much more robust, purposeful, and impactful.1,4 While NICE and IQWiG recognize the value of patient experts’ involvement in the overall assessment process, including the patient’s experiences with the disease and the given technology, adequate resources and information required for effective PE are minimal.4,16 Patients and their representatives often have to rely on publicly available data, clinical trial information, physicians, other patient organizations, existing HTA reports, conferences, media, or internet searches for information on the technology being assessed.4
Conclusions
Patients and patient representatives anticipate their involvement early in the HTA process with a focus on long-term sustained involvement throughout all phases of the HTA process as opposed to discrete engagement events.6,7,10-12 Specifically, patients and patient representatives prefer to be involved in all HTA phases such as defining objectives and problems; identifying and prioritizing technologies for evaluation; assessing ethical, social, and economic issues; and developing a patient-friendly version of the results for public consultation, dissemination, and documentation.11 The gaps in the existing guidelines reveal that the burden is on HTA bodies to ensure that patients and patient representatives are included and have access to the requisite resources for effective participation in the HTA process. Considering the time and resource constraints experienced by patients and their representatives, there is a need for HTA agencies to provide adequate training, establish effective communication channels, and increase transparency to enhance and facilitate effective PE in the HTA process.4,8,9,11 It is imperative that HTA bodies in Europe work together with patients and patient organizations to address these challenges and create a shared vision for enhanced PE throughout the HTA process.
References
1. Hunter A, Facey K, Thomas V, et al. EUPATI guidance for patient involvement in medicines research and development: health technology assessment. Front Med. 2018;5:231. doi:10.3389/fmed.2018.00231
2. Single AN, Facey KM, Livingstone H, Silva AS. Stories of patient involvement impact in health technology assessments: a discussion paper. Int J Technol Assess Health Care. 2019;35(4):266-272. doi:10.1017/S0266462319000552
3. EUnetHTA. Joint Action on HTA 2012-2015: HTA Core Model Version 3.0. Accessed June 1, 2022. https://www.eunethta.eu/hta-core-model/
4. Scott AM, Wale JL, On behalf of the HTAi Patient and Citizen Involvement in HTA Interest Group PIaEWG. Patient advocate perspectives on involvement in HTA: an international snapshot. Res Involv Engagem. 2017;3(2):1-7. doi:10.1186/s40900-016-0052-9
5. Cavaller-Bellaubi M, Faulkner SD, Teixeira B, et al. Sustaining meaningful patient engagement across the lifecycle of medicines: a roadmap for action. Ther Innov Regul Sci. 2021;55(5):936-953. doi:10.1007/s43441-021-00282-z
6. Gunn CJ, Bertelsen N, Regeer BJ, Schuitmaker-Warnaar TJ. Valuing patient engagement: reflexive learning in evidence generation practices for health technology assessment. Soc Sci Med. 2021;280:114048. doi:10.1016/j.socscimed.2021.114048
7. Hameen-Anttila K, Komulainen J, Enlund H, et al. Incorporating patient perspectives in health technology assessments and clinical practice guidelines. Res Social Adm Pharm. 2016;12(6):903-913. doi:10.1016/j.sapharm.2015.12.005
8. Hayes K, Hernon M, Dooley B. Barriers and enablers to patient engagement in the health technology assessment process: an Irish perspective. Value Health. 2019;22(3):S828-S829. doi:10.1016/j.jval.2019.09.2277
9. Janssens R, Russo S, van Overbeeke E, et al. Patient preferences in the medical product life cycle: What do stakeholders think? Semi‑structured qualitative interviews in Europe and the USA. Patient. 2019;12(5):513-526. doi:10.1007/s40271-019-00367-w
10. Messina J, Grainger D. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199-211. doi:10.1007/BF03262492
11. Toledo-Chávarri A, Alvarez-Perez Y, Triñanes Y, et al. Toward a strategy to involve patients in health technology assessment in Spain. Int J Technol Assess Health Care. 2019;35(2):92-98. doi:10.1017/S0266462319000096
12. Whichello C, van Overbeeke E, Janssens R, et al. Factors and situations affecting the value of patient preference studies: semi-structured interviews in Europe and the US. Front Pharmacol. 2019;10(1009)doi:10.3389/fphar.2019.01009
13. Faulkner SD, Sayuri Ii S, Pakarinen C, et al. Understanding multi-stakeholder needs, preferences and expectations to define effective practices and processes of patient engagement in medicine development: a mixed-methods study. Health Expect. Apr 2021;24(2):601-616. doi:10.1111/hex.13207
14. Gesbert C, Andre-Vert J, Guerrier M, et al. The contribution of French patient and consumer groups to health technology assessments over a 2-year period: an observational retrospective study. Int J Technol Assess Health Care. 2021;37:e48. doi:10.1017/S0266462321000180
15. Stewart JA, Clifton E, Macpherson K, Angelova N, Morrison G. Scottish Health Technologies Group: enhancing patient engagement. Int J Technol Assess Health Care. 2021;37:e21. doi:10.1017/S026646232000224X
16. National Institute for Health and Care Excellence (NICE). NICE health technology evaluations: the manual. Accessed June 1, 2022. www.nice.org.uk/process/pmg36
17. Institute for Quality and Efficiency in Health Care (IQWiG). General Methods Version 6.1. Accessed June 1, 2022. https://www.iqwig.de/en/about-us/methods/methods-paper/
18. Agencia Española de Medicamentos y Productos Sanitarios. Committee for Medicinal Products for Human Use (CMH). Accessed March 15, 2023. https://www.aemps.gob.es/la-aemps/comites-tecnicos-de-la-aemps/comite-de-medicamentos-de-uso-humano-cmh/
19. Spanish Network of Agencies for Assessing Health Technologies. Decisional flowchart for invovlement in health technology assessments. Accessed June 1, 2022. https://redets.sanidad.gob.es/en/productos/buscarProductos.do?metodo=detalle&id=1096
20. Haute Autorite de Sante (HAS). General method for assessing health technologies. Accessed June 1, 2022. https://www.has-sante.fr/jcms/c_2035673/en/assessment-of-health-technologies-and-procedures#toc_1_1_1
21. Agencia Italiana Del Farmaco (AIFA). Open AIFA. Accessed June 1, 2022. https://www.aifa.gov.it/en/open-aifa1
22. European Patients Forum. PARADIGM. Accessed August 24, 2023. https://www.eu-patient.eu/projects/completed-projects/paradigm/
23. European Network for Health Technology Assessment (EUnetHTA). HTA Core Model User Guide Version 1.1. Accessed June 1, 2022. https://www.eunethta.eu/wp-content/uploads/2018/06/HTACoreModel_UserGuide_Version1.1-1.pdf

