Good Publication Practice: Values for Health Economics and Outcomes Research Professionals
Lisa De Tora, PhD, MS, Hofstra University, Hempstead, NY, USA; Tom Drake, MA, BS, Global Outcomes Group, Reston, VA, USA; Laura Dormer, BSc, Becaris Publishing Ltd, Royston, England, UK
Introduction: The Importance of HEOR and RWE
Health economics and outcomes research (HEOR) and real-world evidence (RWE) provide valuable insights into both economic and health outcomes for healthcare interventions, considering cost-benefit and budget implications. These data provide clinicians with an enhanced understanding of effectiveness, safety signals across diverse patient groups, and possible impacts on a population basis for various demographic groups. Such findings are also used for health technology assessments (HTAs), formulary decisions, regulators, and funding bodies. Thus, HEOR and RWE experts routinely publish research results and may encourage the registration of RWE studies in the ISPOR RWE registry.
ISPOR has shown a commitment to the continual improvement of publications and communicating research findings where they are needed. Groups such as ISPOR RWE Task Forces do important work to bring these findings to those who need them, including patients and other nontechnical decision makers. Individual experts also play important roles in publications. Like subject matter experts in any biomedical field, HEOR and RWE professionals may serve as authors, advise colleagues on research design or publications, and review such work within research teams or as peer reviewers (Table 1). For research about marketed or investigational medicinal products or other collaborations with industry sponsors, additional logistical and ethical considerations may affect this work.1,2 For these reasons, the Good Publication Practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 Update1 was designed to apply across scientific areas and is now more compatible with HEOR and RWE publications as a general rule. Assisting a wide range of healthcare stakeholders, GPP 2022’s acknowledgement of HEOR and RWE as integral to biomedicine supports value-based decision making across all levels of formulary approval and resource utilization and helps promote the ongoing work of ISPOR members in improving publication quality.
We provide a high-level overview of GPP and its application for HEOR and RWE audiences with an emphasis on the increasing involvement of patients, caregivers, and patient advocates in research publications.3 Efforts to expand the reach of research findings also include a rise in the publication of plain language summaries (PLS) , often as a “short synopsis of a piece of research presented in a way that is accessible to a broad readership, including nonspecialist healthcare professionals and lay audiences, including patients.”4 HEOR and RWE experts are important collaborators who have an important part to play in enhancing and forwarding these publication practices.
Table 1: Possible Roles for Subject-Matter Experts Related to Publications of Company-Sponsored Research

Good Publication Practice
The Need to Expand
GPP was originally designed to parallel good clinical practice and consequently focused on publications of clinical trials, especially randomized controlled trials.1,2 Through the influence of the International Society of Medical Publications Professionals (ISMPP), GPP became incorporated into an ever-increasing number of publication policies at sponsoring companies and medical communications agencies.
Over time, however, it became evident that attention to additional scientific areas was needed, given the extent to which subject-matter experts from outside clinical research engaged with publications. One specific strategy for updating GPP 2022 was to acknowledge that biomedical research extends beyond the clinical trial and that HEOR and RWE are important scientific areas that required more explicit inclusion. By removing many specific references to clinical publications and mentioning HEOR and RWE wherever applicable, GPP 2022 pulled these areas into the mainstream. Integrating all stakeholders into GPP 2022 was intended to help prevent situations in which HEOR and RWE become a special case or an afterthought.
"GPP 2022’s acknowledgement of HEOR and RWE as integral to biomedicine supports value-based decision making across all levels of formulary approval and resource utilization."
However, it is noteworthy that the general activities associated with scientific specialties like HEOR (Table 1) also apply to patients, caregivers, and patient advocates, who “should be regarded as experts who may give important input into publications.”2 While GPP emphasizes the value of many diverse stakeholders and acknowledges HEOR and RWE as an integral part of the publication landscape, it also recommends that patients, caregivers, and patient advocates be included in publications whenever possible, particularly for programs associated with rare or chronic conditions.
Overview and New Developments
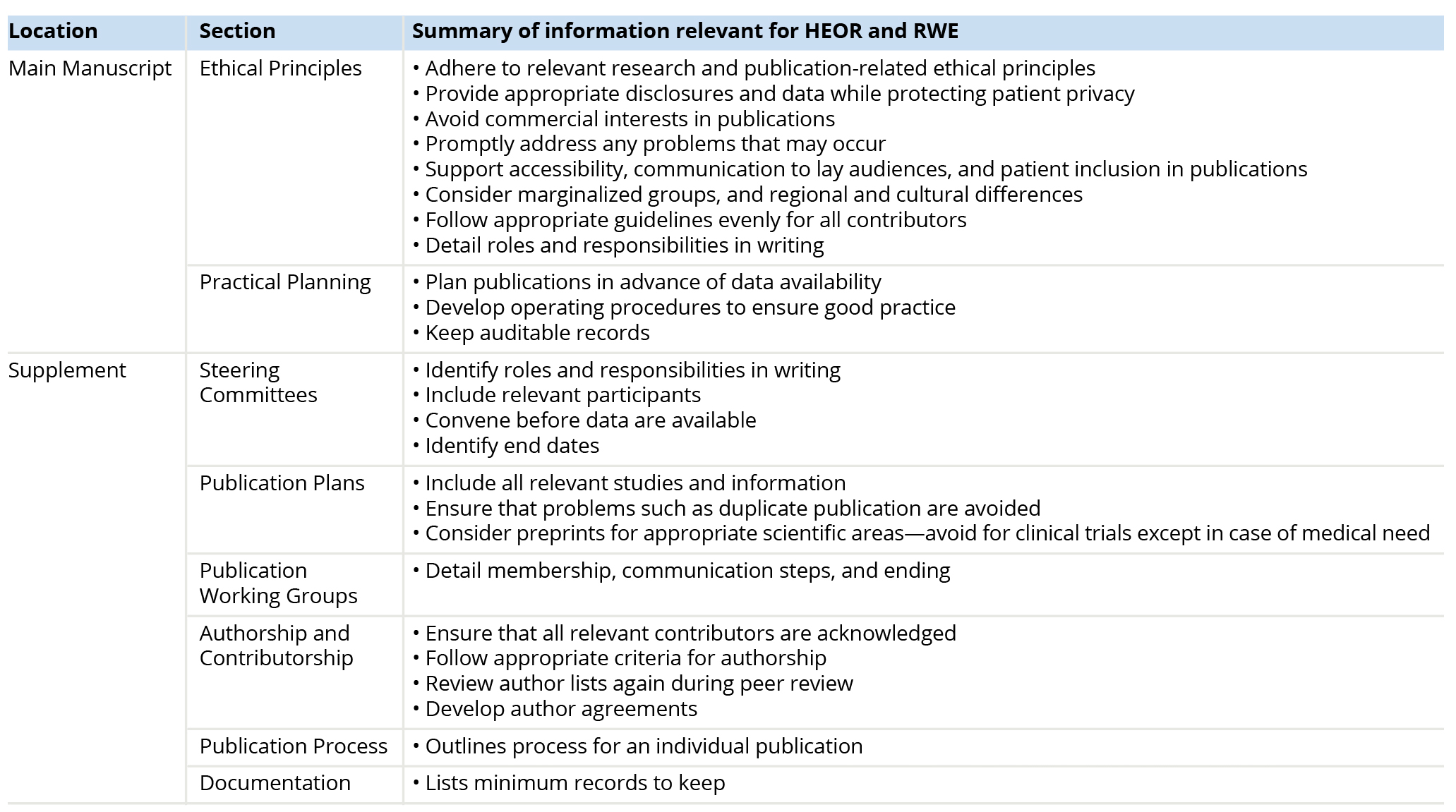
GPP 20222 presents ethical and practical planning principles for biomedical publications in the main text; a detailed supplement presents further information and guidance about day-to-day practices, all of which may apply to HEOR and RWE colleagues and research. Table 2 provides a list of some key considerations for HEOR and RWE professionals and where to find them in GPP 2022.
New developments in GPP 2022 reflect increasing efforts in the past few years to make the outputs of scientific and medical research accessible to a broader audience than its traditional readership. This practice has obvious applications for RWE and HEOR research, as reflected in part by the work of the ISPOR-ISPE-Duke Margolis RWE Transparency Initiative, as well as for increased involvement from patients, caregivers, and patient advocates. GPP 20222 is the first such guidance to present information on PLS and other enhanced content—which might include infographics or videos—that is published as part of the publication.
Table 2: Good Publication Practice Contents of Interest to HEOR Professionals
GPP 2022 recommends developing a PLS for all types of research. For example, text-based PLS are recommended to be submitted with any clinical trial publications following the CONSORT guidance and for “any other publication of clinically relevant information about any currently marketed product.”2 Standalone PLS are another option that may be relevant for HEOR and RWE studies.2 Further contributions are needed from groups such as ISPOR to refine general practice regarding PLS, develop practical models, and continue their efforts in the ongoing improvement of the biomedical publication landscape.
Experts in HEOR and RWE are at the vanguard of several initiatives in company-sponsored research, such as increasing transparency and the involvement of patients in the publication process.
"New developments in GPP 2022 reflect increasing efforts in the past few years to make the outputs of scientific and medical research accessible to a broader audience than its traditional readership."
The benefits of patient engagement in developing medicinal products from the preclinical phase onward have been discussed in numerous publications, and efforts to define best practices are ongoing.5,6 While early attention focused on clinical trials, the value that patient engagement brings to HEOR and RWE is also now being recognized, for example with funding body PCORI’s Public and Patient Engagement program.7 While patients and their caregivers are a clear audience to benefit from PLS, where they can help support patient involvement HTA and HEOR,8 there are numerous other audiences to whom they provide value, including regulatory bodies and payers.9
GPP: Opportunities for HEOR and RWE Leadership
Aside from the obvious need for experts to author their own research, HEOR and RWE experts should be involved across additional publications activities, as appropriate to the research stage of a program or product. For instance, steering committees advise teams on how and where to publish research and whether enhanced content may be useful for specific datasets.2 Although a steering committee may be helpful for larger projects within a specific HEOR and RWE remit, a specialized HEOR/RWE steering committee may provide advice across clinical programs.2 Another option is for individual HEOR and RWE experts to serve within various steering committees for applicable clinical development programs. Steering committee membership generally brings with it the responsibility for contributing to and reviewing publication plans or specific publications.2 As with steering committees, publication plans might exist to cover the HEOR and RWE remit, or individual applicable HEOR and RWE publications might be included within overarching plans for specific products or programs.2
"The inclusion of both HEOR and RWE publications within the GPP framework illustrates the maturing field of value communications and the expanding role of healthcare decision makers."
Another consideration relevant to HEOR and RWE colleagues is in the attribution of authorship. GPP 2022 recommends that all criteria for contributors and authors should be applied evenly to all colleagues, as consistent with journal and conference guidelines. Author agreements are recommended and might be applicable to HEOR and RWE experts as well as clinical investigators.2
Best practices for working with medical writers are also reflected in GPP 2022.2 Given that HEOR and RWE experts may serve as the lead writers for their publications, processes used by professional writers may provide a helpful context or labor-saving information. As HEOR and RWE experts are included in more publication’s teams, they may be called on to work with professional writers.
For many topics, GPP 2022 provides a high-level overview as well as helpful references that can be used to inform practice on individual teams.
Conclusions
The GPP 2022 expanded in important ways by recognizing the need to include HEOR and RWE peer-review publications. This new inclusion provides significant recognition of the value these publications offer to scientific literature and coincides with the US Food and Drug Administration’s ongoing expansion of the acceptance of HEOR and RWE data for their evaluation and approvals of drugs and devices.
GPP 2022 recognizes the importance of RWE publications, which draw insights from real-world data, reflecting efficacy results and side-effect signals from interventions in medical practice. The inclusion of both HEOR and RWE publications within the GPP framework illustrates the maturing field of value communications and the expanding role of healthcare decision makers.2 Published evidence-based data, both economic and clinical, guide resource utilization for healthcare authorities, both private and at the federal, state, and local government levels. The recent pandemic shed light on the importance of real-world data to improve delivery and mitigate significant discrepancies within our nation’s healthcare sector. That the GPP 2022 update openly acknowledged HEOR and RWE publications is a testament to our evolving need for a wider set of evidence and economic analysis tools in medical research and practice management to support best practices in public health and resource utilization.
References
1. DeTora LM, Toroser D, Sykes A, et al. Good Publication Practice (GPP) Guidelines for Company-Sponsored Biomedical Research: 2022 Update. Ann Intern Med. 2022;175(9):1298-1304.
2. Battisti WP, Wager E, Baltzer L, et al, International Society for Medical Publication Professionals. Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3. Ann Intern Med. 2015;163(6):461-464.
3. Oliver J, Lobban D, Dormer L, Walker J, Stephens R, Woolley K. Hidden in plain sight? Identifying patient-authored publications. Res Involv Engagem. 2022;8(1):12. doi: 10.1186/s40900-022-00346-w. PMID: 35410628; PMCID: PMC8996208
4. Lobban D, Gardner J, Matheis R, ISMPP PLS Perspectives Working Group. Plain language summaries of publications of company-sponsored medical research: what key questions do we need to address? Curr Med Res Opin. 2022;38(2):189-200. doi: 10.1080/03007995.2021.1997221. Epub 2021 Nov 4. PMID: 34736362
5. Deane K, Delbecque L, Gorbenko O, et al. Co-creation of patient engagement quality guidance for medicines development: an international multistakeholder initiative. BMJ Innov. 2019;5(1):43-55. doi: 10.1136/bmjinnov-2018-000317. Epub 2019 Mar 2. PMID: 31645992; PMCID: PMC6792320
6. Feldman D, Kruger P, Delbecque L, et al; Patient Focused Medicines Development Working Groups 1; Patient Focused Medicines Development Working Groups 2A; Patient Focused Medicines Development Working Groups 2B. Co-creation of practical “how-to guides” for patient engagement in key phases of medicines development-from theory to implementation. Res Involv Engagem. 2021;7(1):57. doi: 10.1186/s40900-021-00294-x. PMID: 34425911; PMCID: PMC8383358
7. PCORI. Public and Patient Engagement. Accessed July 2, 2023. https://www.pcori.org/about/about-pcori/our-programs/engagement/public-and-patient-engagement
8. Harvey E, Blumer Z, Carthy J, et al. PNS252 Patient involvement in health technology assessment and health economics and outcomes research: rapid evidence assessment and interviews on the use of plain language summaries. Value Health. 2020;23(2):S683.
9. Cook N, Livingstone H, Dickson J, et al. Development of an international template to support patient submissions in health technology assessments. Int J Technol Assess Health Care. 2021;37(1):e50. doi: 10.1017/S0266462321000167. PMID: 33789779

