Will COVID-19 Accelerate the Adoption of Digital Health Technologies?
Nazneen Fatima Shaikh, BPharm, DPharm, Department of Pharmaceutical Systems and Policy, West Virginia University, Morgantown, WV, USA; Purva Parab, BPharm, MS, Virginia Commonwealth University, Richmond, VA, USA
Session moderator Kristian Kidholm, PhD, Center for Innovative Medical Technologies, University of Southern Denmark, Odense, Denmark opened this issue panel discussion by emphasizing the widespread use of digital technologies. He mentioned that with the pandemic there is a big need to find innovative ways to deliver healthcare. Telemedicine has become increasingly important in light of COVID-19 because it leverages digital technologies to enable healthcare providers to consult with patients remotely. Some examples of these digital tools include telephone/video consultation with patients, apps with information and text messages, home monitoring of clinical data from patients, and home treatment of patients through the use of digital devices such as pumps for intravenous injection of antibiotics. In this session, Kidholm and the panelists discussed ways to evaluate and assess value of digital healthcare.
Using Randomized Controlled Trials to Evaluate Digital Healthcare
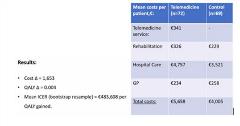
Maja Rasmussen, MS, Centre for Innovative Medical Technology, University of Southern Denmark, Odense, Denmark, presented a randomized controlled trial (RCT) approach to digital health. Her group developed and tested individualized cardiac telerehabilitation to reduce mortality as a part of their Teledi@log Project. They assessed the costs and cost utility of the intervention using an RCT approach and evaluated the incremental cost-effectiveness ratio (ICER). A mean ICER of €483,608 per quality of life year was gained suggesting that telerehabilitation was not cost-effective on a small scale (Figure 1). Rasmussen mentioned that the participation rates were lower and due to the study design long-term outcomes could not be studied. Therefore, a larger sample and longer studies would be required to assess the effectiveness of this intervention.
Figure 1. RCT Approach for Evaluating Digital Healthcare.
Alternatives to Randomized Control Trials
Jason Shafrin, PhD, PRECISIONheor, Los Angeles, CA, USA, discussed evaluating digital health technologies in the absence of RCTs. “With COVID happening, there is more and more interest in digital health,” Shafrin said. Examples of digital medicine that are used in healthcare are summarized in Figure 2.
Figure 2. Examples of Digital Medicine.

Without an RCT, models can be used to combine surveys and real-world evidence and literature can be used to estimate treatment value. A survey-based approach used to evaluate the effectiveness of an adherence measure technology tool (PDAI – Patient Drug Adherence Information) suggested that more providers who had access to PDAI switched their patients to long-acting injections to increase adherence. There were also annual cost savings per patient when providers had access to PDAI (Figure 3). Model-based approaches can also be used to measure the value of diagnostics and faster point-of-care testing, which is essential given the COVID situation. Shafrin noted that flexibility, lower cost, and long-term outcomes are a few of the benefits of a non-RCT approach.
Figure 3. Survey-Based Approach Model Results.

Taking a Health Technology Assessment Approach to eHealth
Hans C. Ossebaard, PhD, National Health Care Institute (Zorginstituut Nederland), Diemen, The Netherlands talked about the HTA approach to eHealth. Assessment frameworks for evaluating eHealth technologies vary greatly in indicators and methods used and standardization is needed in these techniques. Real-world applicability of these technologies is limited. To accelerate adoption of digital health technology especially in the COVID era, factors such as reduced waiting time, lesser traveling, and less infection risk need to be incorporated in the model.
"Model-based approaches can also be used to measure the value of diagnostics and faster point-of-care testing, which is essential given the COVID situation."—Jason Shafrin, PhD
When Is RCT Needed?
After the individual presentations, Kidholm asked the panelists when they felt an RCT would be necessary. Rasmussen said it would largely depend on the cost and effectiveness of the new intervention. She mentioned that if the intervention has a large effect on clinical outcomes then you might want to go for RCT, but if the patients are also seen in an outpatient setting, then an RCT might not be necessary. Her team also used surveys and patient-reported outcomes to evaluate treatment for which data was collected through apps. Shafrin supported the statement by adding that if apps are replacing pharmaceuticals, then an RCT might be needed. Ossebaard noted that oftentimes informational technology can provide sufficient insights where answers are very evident and a full blown RCT may not be needed.
Shafrin’s final remarks cautioned that some digital health technologies are not integrated in electronic health records. The pandemic has made this disconnect more apparent and will likely be an area that will evolve a great deal in light of COVID.
Explore Related HEOR by Topic

