Patient and Public Involvement in Healthcare Decision Making: Are We Maximizing Opportunities?
Nazneen Fatima Shaikh, BPharm, DPharm, Department of Pharmaceutical Systems and Policy, West Virginia University, Morgantown, WV, USA; and Purva Parab, BPharm, MS, Virginia Commonwealth University, Richmond, VA, USA
Patient and public involvement in healthcare is an increasingly important and positive aspect of the healthcare system. Session moderator, Bettina Ryll, MD, PhD, Melanoma Patient Network Europe, and Past Chair of the Patient Advocates Working Group, European Society for Medical Oncology, Uppsala, Sweden, kicked off the plenary session by pointing out how flawed the healthcare system would be without involving patient needs in decision making. Patients, patient advocates, and specialized/ professional technical experts constitute different domains of healthcare decision making, she said, and there needs to be a balance and involvement from all these groups for an efficient healthcare system.
Methodological Inputs for a Sophisticated Process
Methodological considerations for patient involvement in decision making was discussed by panelist Axel Mühlbacher, PhD, MBA, Hochschule Neubrandenburg, Neubrandenburg, Germany. Healthcare decisions involve a lot of information asymmetries from the patient’s and public’s end, he said, which is why we delegate decision powers to the experts, allowing them to make value judgments based on evidence. This value judgement should, however, also include patient preferences, he added.
“Measuring clinical effect sizes is necessary, but not sufficient for rational healthcare decision making.”—Axel Mühlbacher, PhD, MBA
An example of a decision-making model for a nonpharmaceutical intervention using the COVID-19 pandemic (lockdown, lockdown light, or no lockdown) was provided. The decision model (Figure 1) summarizes various criteria that would be considered while making this decision and the impact of the decision on outcomes such as individual income and excess mortality. However, there is a lot of uncertainty involved in measuring these outcomes and trade-offs within the criteria. For instance, how much personal data would you be willing to give to have a less of a decrease in annual income?
Figure 1. Decision Model for Nonpharmaceutical Interventions.
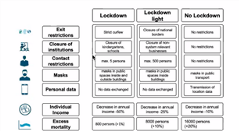
To reduce these uncertainties, utility curves can be used to quantify these trade-offs. These utility curve values should also incorporate patient preferences. In an ideal situation, the different choices and scenarios under consideration can be provided in a discrete choice experiment to patients and the public to provide their inputs. Patients should be able to weigh the decision criteria based on their perception. Mühlbacher then pointed out certain future considerations for evidence-based advocacy that include incorporating data on patient preferences, informing value judgements, and making transparent decisions by having patient involvement.
Involvement of Patient Advocacy Groups
Yann LeCam, Eurordis, Rare Diseases Europe, Paris, France, gave his perspective on patient engagement in healthcare decision making through the advocacy groups for rare diseases. He provided an example using recent COVID-19 vaccines and the necessity of the public’s trust in the information and the data to increase adherence. It is essential to have information on public views to assess the extent of information asymmetry involved. He said, “Patient advocacy groups should not just be able to use their own personal experience but also think about the whole disease group.” Healthcare should move progressively to patient involvement throughout the product cycle, he continued. Patients and the advocacy groups should be involved in developing registries, determining new clinical end points, as well as new patient reported outcomes, said LeCam. In HTA, patient engagement in early scientific dialogue should be incorporated through patient advocacy groups. LeCam emphasized the urgent need for more patient and public involvement in decision making and striking a sense of partnership between patient and public rather than treating them as separate entities. “We have to move from involvement, to engagement, to partnership between public and patient,” he concluded.
“Today’s public can become tomorrow’s patient or caregiver.”—Yann LeCam
Patient Engagement: The EMA Experience
Nathalie Bere, European Medicines Agency (EMA), Amsterdam, The Netherlands provided some insights on her experience with patient engagement at the EMA. “A patient’s voice is an integral element of healthcare decision making. The EMA has seen increasing and varied involvement from patients and the patient advocacy groups throughout different activities and organizations,” she said. (Figure 2). The biggest milestone that EMA had was having patients as voting members on various committees and organization boards. The EMA also involves young people in addition to adults on their patient engagement groups. Emphasis was placed on some key learnings for successful patient engagement that included creating a diverse group of stakeholders, testing and implementing various engagement methodologies, providing appropriate training and support, and being transparent by sharing relevant information.
Figure 2. Patient Engagement With EMA.

Removing Barriers and Increasing Patient Engagement
The panelists (Figure 3) discussed barriers to patient engagement, addressing the conflict of interest issue due to industry funding, and their thoughts on creating robust validated decision-making tools that can be used across disease areas. Mühlbacher addressed the barrier of patients and patient advocates not being fully equipped with scientific data. This makes it easier to influence the patients and the process thus needs to be made more transparent. Influencing patients also leads to reduced validity of the decision-making tool. Mühlbacher mentioned that there are multiple methods that are discussed in the literature to create a robust validated tool and these need to be standardized to be applicable across disease areas.
Figure 3. Panelists (clockwise) Nathalie Bere, Bettina Ryll, Yan LeCam, Axel Mühlbacher.
Bere emphasized that language barriers and time management issues with patient involvement can be addressed by conducting smaller pilot studies. As for conflict of interest, LeCam provided his perspective on reducing this concern by increasing collaboration not just from the industry but from other governing bodies as well. In addition, patient groups can strive to take equal funding from industry and other sources to reduce conflicts of interest. The session concluded by all the panelists recognizing the need for increased patient and public engagement in healthcare decision making.

