Multinational HTA: Does IT Really Benefit Public Health and Promote Access to Innovation?
Aakash Bipin Gandhi, BPharm, Department of Pharmaceutical Health Services Research, University of Maryland, Baltimore, MD, USA; and Vasco Pontinha, MPharm, MA, Department of Pharmacotherapy and Health Outcomes, Virginia Commonwealth University School of Pharmacy, Richmond, VA, USA
“Multinational health technology assessment (HTA) can help increase the efficiency of drug approval processes to ensure quick and equitable access to novel pharmaceutical products.” With this statement, Gavin Outteridge, MA, AESARA Europe, London, United Kingdom, kicked off an exciting issue panel for day 3 of the ISPOR Europe 2020 conference. Panelists from diverse fields, including members of multinational HTA bodies, pharmaceutical industry, and consulting groups (Figure 1) provided an overview of the current state of multinational HTA in Europe and highlighted steps that can be taken to ensure they continue to have a positive impact on public health.
Figure 1. Panelists (clockwise): Vanessa Schaub, Gavin Outeridge, and Finn Børlum Kristensen.
Outteridge remarked that, “While the promise of multinational HTA remains, the lived experience falls short.” Ideally, multinational HTA should help provide faster access to drugs for patients, increase the efficiency for the preparation and conduct of HTA assessments, and promote broader public health equity. However, currently, multinational HTA results in the same or slower decision making, leads to new assessments being layered on top of old ones, and causes unreconciled differences in health systems with regards to methods used for value assessments. Hence, while multinational HTA seems promising, there is a long way to go with these assessments.
“While the promise of multinational HTA remains, the lived experience falls short.” —Gavin Outteridge, MA
Finn Børlum Kristensen, MD, PhD, Danish Centre for Health Economics, University of Southern Denmark, Hillerød, Denmark shed light on the HTA process. First, HTAs entail a scoping step that requires the identification of key questions to be answered and output required from the process. Second, the assessment step includes the identification, analysis, and scientific interpretation of research information. The third and final step of contextualization or appraisal involves the application and use of assessment results. According to Kristensen, such processes can be achieved at a global level, provided that the one is able “to locate the decision, globalize the evidence, and localize the reporting of results.”
“The issue has to do with the lack of a central regulatory decision that can guide the efficient uptake of these assessments.” —Finn Børlum Kristensen, MD, PhD
To date, even with the Joint Action Plan 3 agreed upon by EUnetHTA (the European Network for Health Technology Assessment, Link: www.eunethta.eu), implementation of HTA models based on stakeholder cooperation are difficult to implement. In fact, the EUnetHTA WP7 Final Report identified a need for central support that meets the needs of the different HTA agencies across Europe. This can help provide a standard framework for assessment needs once it is developed and agreed upon by member countries. The report further stressed the need for significant investments to ensure adequate capacity and expertise to implement HTA processes at a local level. Kristensen indicated that despite several efforts by the EUnetHTA on joint assessments for pharmaceutical products in oncology and other nonpharmaceutical interventions, the uptake of these assessments at a local level falls short of expectations. In medical devices, the uptake is even less, perhaps due to the differences in clinical practice at a local level. The issue, he argued, has to do with the lack of a central regulatory decision that can guide the efficient uptake of these assessments.
“Currently, there seems to be a huge diversity in lag times between marketing approval and reimbursement decisions across EU countries.” —Vanessa Schaub, PhD
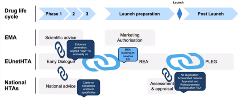
Potential improvements in future revisions of European HTA frameworks were suggested by Vanessa Schaub, PhD, F. Hoffmann-La Roche, Basel, Switzerland. She stated that, “Currently, there seems to be a huge diversity in lag times between marketing approval and reimbursement decisions across EU countries.” For example, the lag times range from around 110 days for Germany to more than 800 days for Poland. This lag time can have critical implications for patients and prevent them from benefiting from innovative treatments. Therefore, Schaube argued for a standardized assessment framework, which may even be pushed via legislation, that at least considers a consistent methodology for HTA across Europe. This can include the use of standard endpoints, agreed-upon subgroups, and consistent use of real-world data. A failure to achieve this methodological consistency will likely result in the failure to implement the multinational HTA at a national level. Schaube pointed out key bottlenecks in the current multinational HTA process, including the need to harmonize early advice from European Medicines Agency and EUnetHTA with respect a local decision (Figure 2). The status quo means duplication of efforts because the early advice on relative effectiveness assessment differs from the evidence required at a local level. Post-HTA assessment and reimbursement decisions also require some form of harmonization. What is the real-world evidence that pharmaceutical companies need to generate? In other words, what is the totality of evidence that needs to be generated to ensure continued access to treatments?
Figure 2. Bottlenecks to Solve for a Successful Multinational HTA Implementation
In a highly participated session, the audience questioned the panelists on whether the notion of globalized evidence was possible or even a positive to achieve. Schaube and Kristensen agreed that the critical aspect for achieving a globalization of evidence is necessary to establish the generalizability of that evidence. In reality, Kristensen argued, generalizability of data is already achieved—EMA conducts clinical assessments and has the power to centrally approve the marketing authorization of drugs. Since the data for marketing approval is very similar for HTA and reimbursement decisions, we can consider making HTA and reimbursement decisions at a central level in the future. In addition, the majority of treatments that are currently submitted to HTA agencies target small groups of patients. According to Schaube, the data that is submitted for HTA agencies is already from different regions because the number of patients in each jurisdiction is so small. Quality standards and access to real-world data at a local level were identified as the cornerstone to achieve meaningful globalized evidence. Finally, the panelists agreed that multinational HTA is here to stay, possibly extended beyond the European context. Whether it is positive or not will depend on cooperation between local agencies, pharmaceutical companies, national regulators, and the healthcare community.

