Optimizing Managed Access: Lessons From the Cancer Drugs Fund
Jennifer Lee, BSc, MBA; Nicola Trevor, MSc, Lola Adedokun, MPharm, MSc, Janssen-Cilag Ltd, High Wycombe, UK; Cameron Lilley, BA, BresMed Health Solutions Ltd, Sheffield, UK
The desire, opportunity, and willingness to increase access to potentially life-saving cancer treatments has mounted in recent years. As the landscape of drug development changes, the criteria for evaluating uncertainty must also change. Some believe the Cancer Drugs Fund (CDF) framework should be evaluated to ensure it can operate to its full potential. In this article, we explore potential changes to both the processes and infrastructure of managed access in England as a means of optimizing access for patients.
What Is the Cancer Drugs Fund?
The government of the United Kingdom established the CDF in 2010 as a mechanism to pay for cancer drugs not yet approved for use by the National Institute for Health and Care Excellence (NICE). The CDF is a key component of managed access in England, giving patients access to promising treatments at lower prices earlier while further data are collected to resolve uncertainties specified by NICE. At its core, the principles are quite simple; following a first assessment, NICE now has an option to make an interim funding decision, a “yes on the CDF,” enabling reappraisal at the end of the data collection period. If the treatment is found to be a cost-effective use of resources, it may be then approved for use via routine commissioning, accompanied by a mandatory funding directive. These routes of commissioning are illustrated in Figure 1. In cases where there is plausible potential for cost-effectiveness but substantial uncertainty, a managed access agreement and further data collection may be agreed upon with the CDF.
Figure 1. Schematic of routes to commissioning in the National Health Service in England for new medicines.
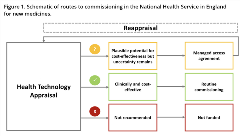
All signs trend towards there being greater reliance on managed access in the coming years. Advances in personalized medicine development bring the promise of important clinical benefits. With these developments, however, come unprecedented levels of uncertainty in the evidence base upon which reimbursement decisions are to be made. Pairing this with increased budgetary pressure means new complexity around how to appropriately manage access to new treatments is unavoidable. The advent of these new technologies is therefore heavily reliant on the willingness of payers to adapt to accommodate them.
The Taxonomy of Uncertainty
Before considering how the current system could be reformed, it is worth commenting on what we mean by “addressing uncertainty.” In the context of NICE, mechanisms to address uncertainty can be categorized under 2 broad headings: (1) effectiveness-based solutions and (2) cost-based solutions.
In its simplest form, in cases where overall survival (a key outcome in oncology) may be immature, longer follow-up from an existing clinical study may increase confidence in modeled long-term estimates. In theory, overall survival data can be collected while the treatment is made available through the CDF, and then used in a reevaluation of cost-effectiveness by decision makers.
If the key area of clinical uncertainty can be resolved by the maturation of existing clinical studies, the current arrangements can continue to be effective. Frequently, however, comparator efficacy is identified as a primary source of uncertainty, but few data collection agreements seek to obtain new comparative evidence.1 Expanding the options for data collection should increase the likelihood that, on reappraisal, key uncertainties identified during the initial appraisal can be appropriately addressed. Figure 2 breaks down the key decision-making criteria considered when evaluating whether a treatment would be eligible for approval via the CDF. In all cases, an a priori assumption that uncertainty can be resolved by further data collection is required.
Figure 2. Cancer Drugs Fund eligibility flow diagram.
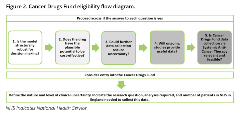
NHS indicates National Health Service.
The other side of the equation is cost, namely drug acquisition cost. Where the evidence base is uncertain, manufacturers may decide to trade off some uncertainty by offering the drug at a lower price. On face value, this appears to be an option with real desirability for NICE, National Health Service England, and society in general, as the burden of that uncertainty is placed almost entirely with the manufacturer. In general, in England, when a treatment is evaluated for use in routine commissioning, different prices are not routinely permitted for different indications of the same drug. By contrast, such price flexibility is encouraged as part of a CDF agreement. This means that for the treatments with more than one indication that are or have been approved for use by the CDF in recent years, the manufacturer has been able to trade off uncertainty for each specific indication while the treatment is available through the CDF. Upon reevaluation such flexibility will cease.
Data-Based Solutions: Is the Systematic Anti-Cancer Therapy Dataset Fit for Purpose?
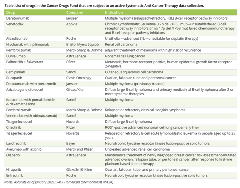
The Systematic Anti-Cancer Therapy dataset (SACT) is mandated as part of the Health and Social Care Information Standards that collects information on using systemic anticancer therapies across all National Health Service England Trusts. In all cases, when a treatment is approved for use via the CDF, data collection via SACT is underwritten in a formal data collection agreement. As is shown in the Table, the list of therapies and disease indications that are subject to active SACT data collection in England is substantial. Some of these treatments will be shown to be cost-effective and become available for routine commissioning upon reappraisal and some will not. While access to these data following a predefined CDF data collection period can help decision makers understand how a drug may be used in practice, SACT has several shortcomings as a primary evidence source. First, complete data collection is limited to few outcomes—primarily overall survival, time-on-treatment, and subsequent treatment. Data on health-related quality of life or even progression-free survival do not often lend themselves to collection in the “real-world” due to differences in reporting and monitoring practices. The development of more diverse and accessible data systems may allow manufacturers and NICE to address a broader range of uncertainties. Second, the burden of data collection in the current system falls to the National Health Service. With the number of treatments approved via the CDF only set to increase, there should be real concerns about this ever-growing administrative burden.
Table. List of drugs in the Cancer Drugs Fund that are subject to an active Systematic Anti-Cancer Therapy data collection.
Alternative data collection models, such as those described by Grieve, et al,2 propose using “only in research” recommendations, encouraging the commissioning of additional, randomized controlled trials to establish relative drug efficacy without the need for observational data and application of advanced statistical methods to form comparisons. Although randomized controlled trials are considered the gold standard for establishing the safety and efficacy of a drug, they do not determine how the drug will perform in real-life clinical practice compared to the standard of care in the National Health Service. This is the main question that NICE is seeking to answer. Moreover, from an ethical perspective, there are conditions where assigning patients to the control arm of a study where they might receive suboptimal treatment should be discouraged.
A middle ground between new randomized controlled trials and routine data collection via the SACT could be a subsidized registry system whereby data collection and access are funded, at least in part, by manufacturers and coordinated in collaboration with methods experts at NICE. The goal of such a system would be to address a broader range of uncertainties, improve clarity in how the data collection will be used to inform reappraisal, and crucially, help ameliorate the administrative burden currently borne by the National Health Service.
Cost-Based Solutions: Buying Out Uncertainty
Improvements in data quantity and quality will take time and decisions will always need to be made in the context of imperfect information. Indeed, in some cases uncertainty may be unresolvable because the time required to generate sufficient evidence may be too long. It is here that an expansion in the approach to drug pricing could be valuable.
One option could be to allow companies to offer different prices across different indications. This would mean that for each indication considered, the manufacturer would have the ability to apply a different price based on the value offered by the treatment in a given indication and the uncertainty that remains in the evidence base.
More routine and efficient commercial flexibility such as the above, alongside schemes such as treatment caps or even outcomes-based agreements could significantly reduce the data collection burden on the National Health Service. Cancer Research UK have themselves taken steps to explore how patients and carers perceive the value of certain health outcomes, the key first step to establishing how such schemes could be implemented.3
While some progress towards commercial flexibility has been made in the National Health Service Commercial Framework for Medicines, this is focused towards medicines at or below the lower end of NICE’s willingness-to-pay range. The reality is that drugs with the potential to be highly effective are struggling to gain access due to uncertainty in the evidence base, and these are unlikely to provide sufficient return on investment at or below the lower end of the willingness-to-pay range. Changes to the system, whether the implementation of new or expansion of current commercial options, must ensure that the implementation of such arrangements minimizes the administrative burden on National Health Service England and front-line staff. In time, the Commercial Framework should pay for itself through rebate systems, but participation must be encouraged.
Looking Ahead
As the drug development pathway changes so should both the CDF and the wider health technology assessment landscape to accommodate those changes and to keep pace with evolving patient needs.
The COVID-19 crisis has highlighted the urgent need to invest in National Health Service data to inform population health. Introducing system-level improvement in real-world data collection, management, and access could contribute to resolving clinical uncertainty in specific populations or societal welfare as a whole. If manufacturers, NICE, and the National Health System can jointly shoulder both the initiative and cost of reform, more opportunities will be unlocked for patients. Introducing formal flexibility for true risk-sharing pricing schemes would allow manufacturers to trade off the uncertainty in the clinical evidence. Doing so would allow NICE to remain innovators in health technology assessment decision making, help establish an appropriate process for which manufacturers and National Health Service England can share risk, while securing continued access to the most promising treatments for patients in England and Wales. Through joint investment in evidence generation infrastructure, research to refine methods of analysis and a willingness to explore alternative approaches to pricing, the capacity for informed decision making greatly increases. While having complex arrangements in place for every treatment assessed is clearly not feasible, more needs to be done to find pragmatic solutions without compromising the timeliness of patients’ access to treatment. •
References
1. Critchlow S, Lilley C, Gladwell D, et al. A Targeted Review Evaluating Uncertainty in Single Technology Appraisal Submissions for Treatments Approved for Use Since the Introduction of the “New” Cancer Drugs Fund. ISPOR Europe 2019; Copenhagen, Denmark. November 2-6, 2019. Poster PCN380.
2. Grieve R, Abrams K, Claxton K, et al. Cancer Drugs Fund requires further reform. BMJ 2016;354:i5090. doi: https://doi.org/10.1136/bmj.i5090.
3. Cole A, Cubi-Molla P, Pollard J, et al. Making outcome-based payment a reality in the NHS. Published February 2019. https://www.cancerresearchuk.org/sites/default/files/obp_final_report_pdf. Accessed January 12, 2020.
Explore Related HEOR by Topic

