Principles to Support Access to Multi-Indication Oncology Combinations
Tim Wilsdon, Charles River Associates, London, England, United Kingdom; Luca Morlotti, Merck, Sharp, & Dohme, Kriens, Switzerland
Introduction
Combinations of new innovative oncology therapies increasingly represent the preferred treatment option for many patients, delivering significant benefits and prolonged survival compared to monotherapies. However, access to branded combinations poses several challenges, particularly where the constituents are produced by different manufacturers and the medicines have multiple indications.
Advances in Treatment
While combination therapies are already used in oncology (such as combinations of chemotherapy treatments), in recent years there have been significant advances in treatment with the introduction of targeted therapies and immunotherapies. Just a few years ago, combination therapies had little effect on progression-free survival and overall survival, now they are beginning to show significantly positive effects; every combination therapy denied to patients could have serious consequences on their health outcomes. Combinations of these new innovative oncology therapies increasingly represent the preferred treatment option for many patients, delivering significant benefits and prolonged survival compared to monotherapies.
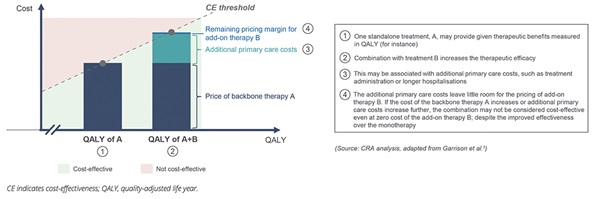
However, access to branded combinations poses several challenges, particularly where the constituents are produced by different manufacturers and the medicines have multiple indications. These difficulties ultimately lead to the “combination challenge”: although a combination would deliver benefits to patients and the healthcare system, and the medicines could be priced in a manner that rewards the companies involved and would represent good value for money for payers, the rigidities in the pricing and reimbursement system prevent patients from gaining access to new, innovative combination medicines. The application of rigid cost-effectiveness can be even more problematic, potentially leading to a phenomenon where a combination may not be cost-effective even when the add-on therapy is priced at zero cost (Figure 1).
Figure 1. Additional costs arising from longer duration of treatment may mean that there is no possibility of cost-effectiveness for a combination treatment, even if the add-on is priced at zero.
During a symposium at the Global ISPOR conference on May 18, 2021, the challenges associated with providing access to oncology combinations, particularly where each constituent is produced by different manufacturers and is present in multiple indications, and the potential approaches to address these challenges were discussed. Tim Wilsdon, Vice President, Charles River Associates, presented a new study that investigated the level of access to combinations in the market today, the challenges affecting branded combination therapies in oncology, and the potential policy solutions. He noted that despite the benefits of combination products (for example, addressing drug resistance and targeting specific patient population subsets), there are challenges to recognizing the value of combinations, especially when constituents may be owned by different manufacturers and may be applicable in multiple indications.
The Challenges Presented by Combination Therapies
To document the impact of the challenges, Charles River Associates performed a numerical analysis. Data on all branded oncology combinations approved in the European Union between January 2015 and October 2020 were collected. The availability of and delay associated with branded oncology combinations was compared to all oncology therapies. A range of stakeholders were also interviewed to validate and support the conclusions.
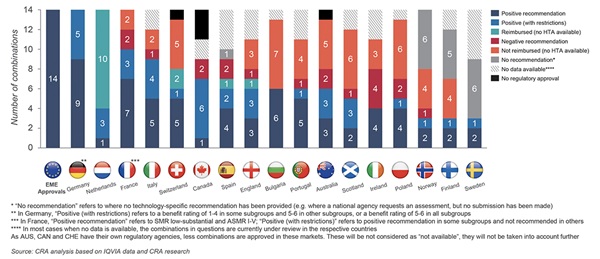
The study looked at the 2020 reimbursement status for each of the 14 oncology combinations approved by the European Medicines Agency during the study period. Figure 2 shows the reimbursement status for the combinations studied—a smaller percentage of combination products were reimbursed, versus noncombination products. Furthermore, when the combination involved constituents from more than one company, a lower proportion were reimbursed compared to when both constituents in the combination were owned by a single company. In addition, the analysis suggests that countries with a cost-effectiveness threshold find it more challenging to reimburse the combination therapies.
Figure 2. The reimbursement status in October 2020 of branded combinations (including both multiple- and single-sponsor combinations) approved in the European Union between January 2015 and June 2020.
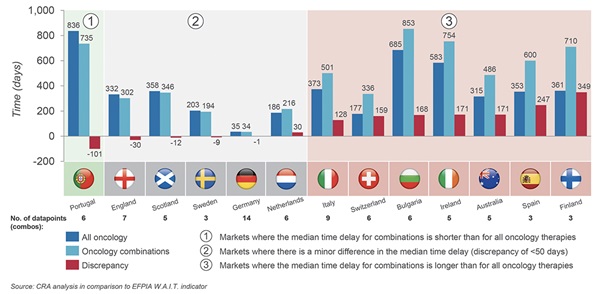
For those combinations that are recommended, the time to reimbursement is longer compared to the average time for all oncology products across the countries included in the study. Figure 3 compares the time to reimbursement for oncology combinations and oncology monotherapies.
Figure 3. Median time between regulatory approval and reimbursement for the combinations (including both multiple- and single-sponsor combinations) in the study that were approved for reimbursement, compared to the same time measurement for all oncology medicines as reported in the EFPIA WAIT indicator.
The qualitative component of the research suggested that the challenge to reimbursing combination therapies is a structural one. Only one of the manufacturers, the one of the new add-on therapy, is involved in negotiations with price and reimbursement authorities regarding the price. It may not be possible for the manufacturer of the add-on therapy to charge a price that results in the combination being cost-effective, even though it offers clinical benefits to the patient. Where rigid cost-effectiveness thresholds are applied, this problem is exacerbated.
There is a range of even more complex challenges: one or more therapies may be launching in their second or more indication, with the manufacturer seeking to avoid price erosion in the primary indication; the combination may compete with one of the constituents; if one constituent is near loss of exclusivity, its incentives for expanding into the combination may be small.
Finding a Way Around the Difficulties to Patient Access
In terms of access and usage, there are also delays to inclusion in clinical guidelines and inappropriate incentives for prescribing combination therapies. Advocates find it very frustrating when a patient cannot access a combination therapy that works a lot better than the monotherapy they have access to. For example, The International Kidney Cancer Coalition is doing its best to educate patient advocates; however, there are therapies that can be cost-effective yet are not getting to patients because of this.
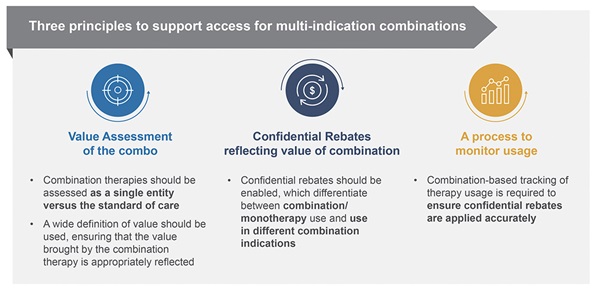
Three principles to support access to multi-indication combinations were proposed (Figure 4): (1) combination therapies should be assessed as a single entity versus the standard of care, including the use of a wider definition of value, ensuring that the value brought by the combination therapy is appropriately reflected; (2) indication-specific confidential rebates should be enabled, which differentiate between combination and monotherapy use and use in different combination indications; and (3) systems should be developed to track the use of medicines in combination in order to enable accurate calculation of the rebates envisaged. Although these principles can be considered universally useful, in order to address the full range of challenges, country-specific solutions will be required.
Figure 4. Three fundamental requirements to enable policy solutions are needed to solve the policy challenge.
Concerns remain that many payers still appear to see the “combinations challenge” as an issue for the future rather than now. Furthermore, so far payers and health technology assessment bodies have tended to assume this is an “industry problem,” whereas it is actually a challenging situation for everyone concerned. It is therefore desirable for all the stakeholders to be engaged in the debate and to work towards a set of solutions.
Payers should recognize that this is causing problems for patient access now and commit to working with industry and other stakeholders on solutions. The actual solution is likely to be location-dependent and needs local engagement because the influence of the law is critical and each country brings its own “remit” and “culture” regarding a solution.
It must also be recognized that the challenge does not end at the reimbursement decision as there remain concerns about usage and uptake. Doctors outside of large centers do not necessarily have the same access to information about combination therapies or how to use them, and these doctors are needed to represent their patients.
Conclusion
As we are moving towards a “combinations” world in cancer treatment, we have the opportunity to achieve better outcomes for patients, turning cancer from a deadly into a manageable disease. However, empirical data show that patient access to combinations faces several hurdles. To date, access issues have been addressed on a product by product basis, but this approach is not sustainable going forward. Given the number of such products in development, these challenges will become a significant issue for patients and society. Certain policy principles could be applied to create the conditions for negotiating sustainable access, in particular an appropriate value assessment, combination-specific rebates, and the tracking of usage. To achieve better access to combinations, a dialogue around these principles between industry, patient advocates, health economists, and authorities is needed.
References
1. Pistollato M, Wilsdon T, Steele R, et al. PCN36 Oncology Combinations: How to Ensure Timely Access? Value Health. 2021;24(supp1):S25.
2. The European Federation of Pharmaceutical Industries and Associations. EFPIA Patients W.A.I.T. Indicator 2019 Survey. Published 2019. Accessed August 21, 2021.
3. Garrison L, Dankó D, Perrier L, Lothgren M. Challenges in the Value Assessment, Pricing, and Funding of Targeted Combination Therapies in Oncology. Issue Panel 17. ISPOR Europe 2018. Accessed August 21, 2021.

