Humanistic Burden of Postpartum Depression in the United States
Poorva Sardana, MSc, Neha Agrawal, MA, Complete HEOR Solutions (CHEORS), Chalfont, PA, USA; Niranjan Kathe, PhD, Amgen Inc, San Diego, CA, USA; Rajender R. Aparasu, PhD, FAPhA, University of Houston College of Pharmacy, Houston, TX, USA
Overview
Approximately 3.6 million live births were recorded in the United States in 2021.1 According to the Centers for Disease Control and Prevention (CDC), 1 in 8 women reported severe and long-lasting symptoms of depression after giving birth,2 representing 450,000 women in 2021. This form of depression after childbirth is a common obstetric complication, termed as postpartum depression (PPD). Current evidence suggests that many biological, social, and psychological factors are responsible for the development of PPD,3 which reportedly occurs in 13% to 20% of new mothers.4 However, there often exists a stigma around appropriate screening measures and missed opportunities by healthcare providers to enquire about depression during prenatal and postpartum visits. Therefore, the actual incidence of PPD is likely to be higher in the United States than the reported estimates.2,5
"There were no specific drugs or pharmacotherapies approved by the US Food and Drug Administration for treating postpartum depression until the approval of brexanolone in 2019."
Due to similar symptoms, PPD is often considered synonymous with another common manifestation of depression following childbirth called “postpartum blues” or “baby blues.” While the latter starts within 1 to 3 days after birth without significantly inhibiting maternal functioning, symptoms of PPD can occur and persist up to a year following childbirth and even result in functional impairment.4 Moreover, in contrast to symptoms of postpartum blues, which resolve with emotional support and reassurance, PPD usually requires interventions such as interpersonal psychotherapy, cognitive behavioral therapy, psychodynamic psychotherapy, medications, or a combination of these.4 The commonly occurring symptoms of PPD are summarized in Figure 1.
Figure 1: Common symptoms of postpartum depression among new mothers

The severity of this condition is reflected in the fact that suicide accounted for approximately 20% of postpartum deaths among new mothers.5 Besides affecting the mothers, PPD also has spillover effects on the welfare of newborns and may adversely affect their behavioral, emotional, and cerebral development in the long-term.5 Therefore, there is substantial morbidity and mortality associated with PPD and a considerable burden from societal and economic perspectives. The treatments for PPD mainly consisted of strategies and therapies similar to the treatment of major depressive disorders,6 and there were no specific drugs or pharmacotherapies approved by the US Food and Drug Administration for treating PPD until the approval of brexanolone in 2019.6 Brexanolone has a multipronged mechanism of action with neuroprotective, anxiolytic, and antidepressant properties. This approval, a transformative breakthrough in the treatment landscape of PPD, has also opened opportunities for further development of more targeted drugs.5
"Based on the diagnoses of mental health conditions or prescription of medications for PPD, 550,268 women (~11%) annually had evidence of PPD."
Few prior studies have examined the quality of life among women with PPD and assessed the effects of this condition on maternal health-related quality of life (HRQoL).7 Moreover, such data for the United States are limited. In this study, the authors have examined the impact of PPD on HRQoL of adult women with childbirth in the United States using a generic quality-of-life instrument for mental and physical health status. The findings of this study can help in understanding the impairment of HRQoL due to PPD and the need to improve the quality of care using targeted treatments.
Study Details
This study used the Medical Expenditure Panel Survey (MEPS) data for the years 2016 to 2019. We identified women aged 18 to 50 years with a childbirth event (ie, diagnosis of pregnancy or delivery or encounter of postpartum examination/lactating women) and no evidence of prior mental health conditions. PPD cases in the study were identified using diagnosis codes associated with depression, mood, or anxiety disorders or drug codes for prescription medications concerning PPD. Two mutually exclusive cohorts, ie, PPD and non-PPD, were created after classifying women with either diagnosis codes or drug codes pertaining to PPD in the former cohort.
Furthermore, the HRQoL of women was examined using short form-12 version 2 (SF-12v2) mental component summary (MCS) and physical component summary (PCS) scores. These scores are components of the SF-12v2 HRQoL instrument and include 12 items capturing information regarding mental and physical health status. The MCS score includes social functioning and mental or psychological health, whereas the PCS score captures limitations in physical activities and pain. The higher the MCS and PCS scores, the better the mental and physical health status.
"The availability of PPD-specific effective treatment approaches may improve not only the quality of life of mothers but also their newborns and families."
This study assessed the effect of PPD on HRQoL while controlling for other characteristics such as age, family size, number of concurrent priority health conditions, education status, marital status, region, smoking status, family income status, race/ethnicity, and survey year. The study cohorts, PPD and non-PPD, were balanced on the characteristics mentioned previously by using propensity score-based inverse probability of treatment weighting (IPTW). Separate linear regression models were used to assess the effect of PPD on MCS and PCS scores. The analysis used appropriate weighting and the complex survey design considerations of the MEPS data to evaluate the relationships and to generate nationally representative estimates.
Findings
The study cohort for this analysis included an unweighted sample of 1873 women aged 18 to 50 years with a childbirth event and no evidence of prior mental health conditions in the United States, representing a weighted sample of approximately 4.3 million women annually. Based on the diagnoses of mental health conditions or prescription of medications for PPD, 550,268 women (~11%) annually had evidence of PPD. These national estimates appear to be consistent with those estimated by CDC.2
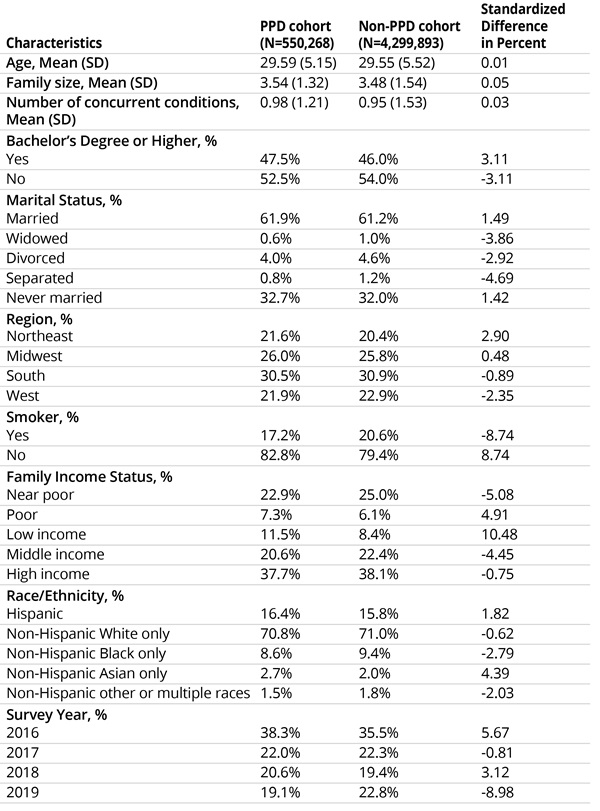
The inverse probability of treatment weights were calculated for the study population using age, family size, number of concurrent conditions, education status, marital status, region, smoking status, family income status, race/ethnicity, and survey year. Furthermore, to adjust for potential confounders, these characteristics were balanced in PPD and non-PPD cohorts using IPTW. Most women with PPD were non-Hispanic White, without bachelor’s degrees, with high family incomes, and nonsmokers. Table 1 depicts summary statistics for study cohorts.
Table 1: Study cohort characteristics
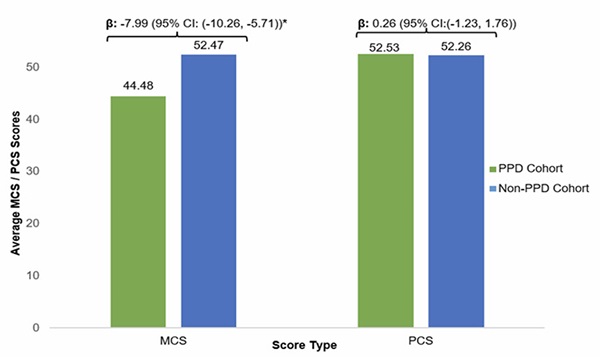
The mean MCS score for women with PPD was 44.48, in contrast to 52.47 for women without PPD. Moreover, the IPTW-adjusted linear regression model indicated that, on average, women with PPD had 7.99 points lower MCS scores than women without PPD. However, there was no significant impact of PPD on physical health status based on the PCS scores (Figure 2). When we converted the MCS and PCS scores to SF-6D utility score8 (data not shown), this translated into a utility difference of 0.07 (0.76 and 0.83 for PPD and non-PPD, respectively), which is higher than the minimal clinically meaningful difference.
Figure 2: Comparison of MCS and PCS scores
Closing Remarks
This study found that women with postpartum depression experience significant and clinically meaningful impairment in their mental health status compared to their counterparts. While women with PPD are also believed to experience changes in physical health status through loss of appetite,4 our study did not find a significant impact of PPD on physical health status using PCS scores. This may be attributed to the lack of sensitivity of the SF-12v2 PCS scale in measuring the impact of PPD symptoms, such as eating habits and weight changes, on physical well-being.
While other studies have assessed the economic burden of PPD on affected households,3,9 our findings present a unique contribution to quantify the humanistic burden of PPD in terms of HRQoL in the US civilian population. Additionally, prior studies have collectively assessed the impact on new mothers and their families,10 but the present study assessed the burden of PPD for the primary affected group (ie, new mothers). However, a few limitations of this study should be noted. First, MEPS is survey data, which comes with inherent biases such as missing data and recall bias. Second, less severe cases of PPD, which may not have required a visit to the healthcare provider, might not have been captured through either diagnosis of mental health conditions or prescription of medications concerning PPD. This indicates that the actual impact of PPD on HRQoL is likely to be underestimated, and further research is needed to assess the impact of PPD based on disease severity.
However, despite the above limitations, our study found that PPD has a significantly adverse impact on the mental health status of new mothers. The availability of PPD-specific effective treatment approaches may improve not only the quality of life of mothers but also their newborns and families.
References
1. Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2021. National Vital Statistics Reports. 2023;72(1):1-53. Accessed May 11, 2023. https://www.cdc.gov/nchs/products/index.htm
2. Centers for Disease Control and Prevention. Identifying maternal depression missed opportunities to support moms. Published online 2020. Reviewed May 2, 2022. Accessed May 11, 2023. https://www.cdc.gov/reproductivehealth/vital-signs/identifying-maternal-depression/VS-May-2020-Maternal-Depression_h.pdf
3. Moore Simas TA, Huang MY, Packnett ER, Zimmerman NM, Moynihan M, Eldar-Lissai A. Matched cohort study of healthcare resource utilization and costs in young children of mothers with postpartum depression in the United States. J Med Econ. 2020;23(2):174-183. doi:10.1080/13696998.2019.1679157
4. Anderson KN, Swedo EA, Trinh E, et al. Parental postpartum depression: more than “baby blues.” Contemporary PEDS Journal. 2018;35(9).
5. Walton N, Maguire J. Allopregnanolone-based treatments for postpartum depression: why/how do they work? Neurobiol Stress. 2019;11. doi:10.1016/j.ynstr.2019.100198
6. Scott LJ. Brexanolone: First Global Approval. Drugs. 2019;79(7). doi:10.1007/s40265-019-01121-0
7. Moore Simas TA, Huang MY, Patton C, et al. The humanistic burden of postpartum depression: a systematic literature review. Curr Med Res Opin. 2019;35(3):383-393. doi:10.1080/03007995.2018.1552039
8. Hanmer J. Predicting an SF-6D preference-based score using MCS and PCS scores from the SF-12 or SF-36. Value Health. 2009;12(6):958-966. doi:10.1111/j.1524-4733.2009.00535.x
9. Epperson CN, Huang MY, Cook K, et al. Healthcare resource utilization and costs associated with postpartum depression among commercially insured households. Curr Med Res Opin. 2020;36(10):1707-1716. doi:10.1080/03007995.2020.1799772
10. Abbasi M, van den Akker O, Bewley C. Persian couples’ experiences of depressive symptoms and health-related quality of life in the pre- and perinatal period. J Psychosom Obstet Gynaecol. 2014;35(1):16-21. doi:10.3109/0167482X.2013.865722

