Looking Beyond Survival Data: How Should We Assess Innovation in Oncology Reimbursement Decision Making
Aikaterini Fameli, PhD, Thomas Paulsson, PhD, GSK, London, England, UK; Shannon Altimari, BSc, GSK, Zug, Switzerland; Ben Gutierrez, PhD, GSK, Collegeville, PA, USA; Ali Cimen, MD, GSK, London, England, UK; Linda Nelsen, MHS, and Nick Harisson, BSc, GSK, Collegeville, PA, USA
ABSTRACT
Traditionally, cancer drugs have been reimbursed based on their ability to extend overall survival (OS). However, in some treatment settings, reliance on OS data presents limitations as it requires long trial durations, is susceptible to confounding from subsequent lines of therapy, and has limited ability to demonstrate impact on disease burden or quality of life (QoL). In settings where time to demonstrate OS is extended, alternative endpoints such as time to disease progression or patient response to treatment can be captured earlier with less confounding and may also have stand-alone value in capturing disease/symptom burden and quality of survival. While payers acknowledge limitations associated with OS, the primary concern preventing adoption of alternative endpoints is uncertainty that these endpoints translate into survival or QoL improvements, thus potentially causing additional treatment burden and cost to healthcare systems without patient benefit. Cross-stakeholder actions to enable greater use of alternative endpoints and patient-reported outcomes (PROs) as measures of value beyond survival in the absence of mature OS data include: (1) ensure sufficient weighting is given to outcomes beyond mortality in health technology assessments (HTAs); (2) improve the PROs toolkit and PROs data collection methodologies; and (3) raise awareness of and generate evidence for the stand-alone value of alternative endpoints. Health economists have a specific role in assessing the socioeconomic impact of drugs that have been approved using alternative endpoints to avoid additional treatment burden and cost to healthcare systems without patient benefit.
Introduction
Cancer is one of the most pressing public health issues of our time. It is the leading cause of death worldwide. In 2020, 10 million people died from cancer, equating to nearly 1 in 6 deaths.1 Furthermore, cancer-related morbidity has a significant and negative impact on patients’ lives; 82% of patients with cancer have low quality of life (QoL)2; pain is prevalent in 55% of patients on anticancer treatment3; and 98% of patients receiving chemotherapy experience severe fatigue.4,5 Beyond its direct impact on patients, families, and carers, cancer is a significant economic burden for healthcare systems, costing more than $21 billion in the United States alone in 2019.6
Cancer treatment has improved significantly. Over the past 40 years, the proportion of patients expected to live for more than 10 years from the time of diagnosis has increased from 20% to 50%.7 Innovation continues to accelerate, with 1500 novel cancer therapeutics in clinical trials as of 2020.8 Rapid scientific advances in cancer treatment, however, are challenging the way access to novel therapies is supported for patients. While regulatory agencies have continued to evolve the criteria for bringing new oncology medicines to patients, the assessment criteria used for reimbursing new therapies in oncology are struggling to keep pace with innovation.
In this article, we review the challenges with the current reimbursement criteria for oncology treatments and discuss the value of alternative clinical trial endpoints and cross-stakeholder actions that can be taken to ensure a future in which alternative endpoints are fit-for-purpose for use in oncology reimbursement decision making.
Overall survival: an endpoint with increasing limitations
Traditionally, cancer drugs have been approved and reimbursed based on their ability to extend patient survival. Overall survival (OS), defined as the time from randomization until death from any cause, has long been considered the gold standard endpoint. OS is a clearly defined and objective measure that reflects unquestionable benefit to the patient. It also enables healthcare systems to conduct direct comparisons of the benefit of new therapies across diseases and to calculate the cost per life year, or quality-adjusted life year as part of a cost-effectiveness assessment. However, there are limitations to using OS as the primary measure of value in reimbursement decisions. These limitations vary by cancer types and stages.
Firstly, the time to demonstrate OS benefit can be extended. In well-managed cancer indications with long baseline survival and/or slow disease progression (eg, chronic lymphocytic leukemia), time to demonstrate OS benefit can take more than a decade.6 In curative settings such as early breast cancer and testicular cancer, survival outlook is even longer and can approach that of the general population.9 Thus, expecting mature OS data for reimbursement of novel therapies in these settings is impractical and may delay access to these novel therapies. As early diagnosis becomes more common in multiple cancer types and treatments become more effective, patients are expected to live longer across all cancer types. Consequently, the issue of long time frames for obtaining OS data will impact more therapies and more tumor types. Developing criteria to measure innovation based on alternative endpoints will be essential to enabling continued patient access to new treatments (Figure 1).
Figure 1. Reliance on OS data for reimbursement decision making presents 3 key limitations
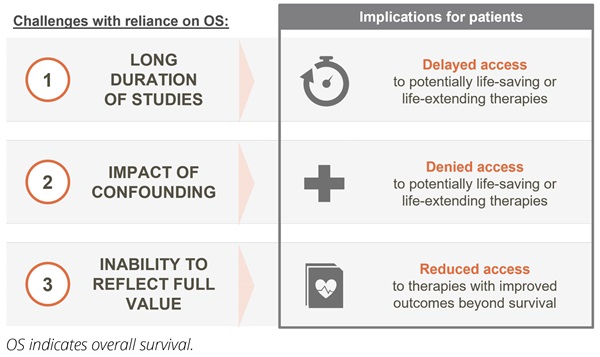
Secondly, OS does not always provide a reliable measure of the efficacy of a new therapy due to its susceptibility to confounding. Confounding, defined as the “mixing of effects,” occurs when the impact of a new drug on survival is mixed in with the impact of additional factors. In early stage cancers, OS data reflect not just the benefit of the novel therapy but also the impact of subsequent therapies that the patient may take as their disease progresses. As the number of later-line therapies across tumor types continues to grow, the ability to demonstrate reliable OS benefit linked to a specific therapy in earlier treatment settings will continue to decrease. Furthermore, aspects of clinical trial design (eg, crossover) may further confound the ability to determine OS benefit.
In addition, OS includes noncancer-related deaths that contaminate the data and reduce the ability to detect the true survival benefit. For example, in patients diagnosed with early breast cancer between 2010 and 2016, more than half of OS events to date have been due to death by causes other than breast cancer.10 In this setting, OS clearly does not provide an accurate measure of the benefit of a new therapy for patients. As cancer care continues to improve, the competing risk of noncancer-related deaths is expected to continue reducing the reliability of OS data across tumor types. Furthermore, even in cancers with shorter survival times, the increasing proportion of patients from older age groups recruited into clinical trials is making it harder to interpret OS data in the face of competing causes of death and old age.11
Finally, there is increasing acknowledgment across stakeholder groups that extending survival should not be the only measure of value. In many cancers, the burden of disease and/or the burden of treatments is high and better disease control, lower morbidity, and fewer side effects can be equally (if not more) important outcomes to patients. Reliance on OS data at the expense of other endpoints and as the primary measure of innovation fails to capture the true value of new treatments to the patient.
The relative importance of value drivers beyond mortality for novel cancer therapies varies by setting. In palliative care, for example, patients often put higher weighting on quality of survival and prioritize being pain free. In a study conducted on the preferences of 459 patients with advanced cancer, when asked to choose between QoL and length of life, 80% of patients chose QoL.12 In early stage, noncurative settings, patients may prioritize OS as an endpoint as they seek to prolong survival. However, even in these settings, the quality of survival will still be important, with the balance between the two dependent on the individual patient.12 Finally, in cancers where survival outlook is very high and the disease is moving toward chronic disease status, extending survival is becoming less relevant as an end goal altogether.
“ The more you are able to make cancer a chronic disease, the less OS is a valid endpoint. That is the balance. HIV is an excellent example and breast cancer is going in the same direction.”
— Clinician (experienced early stage breast cancer physician)
Given the limitations associated with OS, phase III clinical trials in oncology are increasingly relying on alternative endpoints to demonstrate clinical benefit, particularly in early stage disease. Regulators show openness to alternative endpoints. For example, 92% of European Medicines Agency approvals for solid-tumor early stage cancers between 2015-2020 were based on endpoints beyond OS.13 In contrast, reimbursement bodies continue to state a preference for OS data—delaying, denying, and restricting reimbursement of new therapies in its absence.6
Payer preference for OS is driven primarily by the uncertainty around the long-term clinical and economic benefits of new medicines in the absence of mature OS data.6 However, continued reliance on OS is not without consequence. The long time frames to demonstrate OS benefit, the impact of confounding, and the inability of OS to reflect outcomes beyond survival are limiting patient access to innovative therapies. Reliance on OS also leads to indirect healthcare costs, as patients might continue on less efficacious drugs, and reimbursement bodies struggle to identify drugs that lower the overall healthcare burden caused by factors such as patient ability to work, the need for additional lines of salvage therapy, and the burden of care. Finally, an OS-centric approach might reduce incentives for industry investment in certain indications due to high probabilities of failure and discourages researching and developing drugs with lower toxicities and better disease control, thus hindering continued scientific progress.
“ Innovation isn’t innovation without patient access.”
— Patient advocate
Looking beyond survival data: the value of alternative endpointsCommonly used primary endpoints in oncology clinical trials, which are accepted for regulatory approval, include progression-free survival (PFS), defined as the time from randomization to cancer progression or death, and disease-free survival (DFS), defined as the time from randomization to cancer recurrence or death. In addition, scientific progress in the ability to detect the depth of patient response to therapy is leading to increasing interest in the use of endpoints that measure the level of detectable disease following treatment, such as pathological complete response (pCR) and minimal residual disease (MRD). These alternative endpoints, in combination with patient-reported outcomes (PROs), provide the opportunity to address each of the challenges associated with reliance on OS data (Figure 2).
Figure 2. Alternative endpoints (beyond OS) and PROs provide the opportunity to address the limitations associated with OS
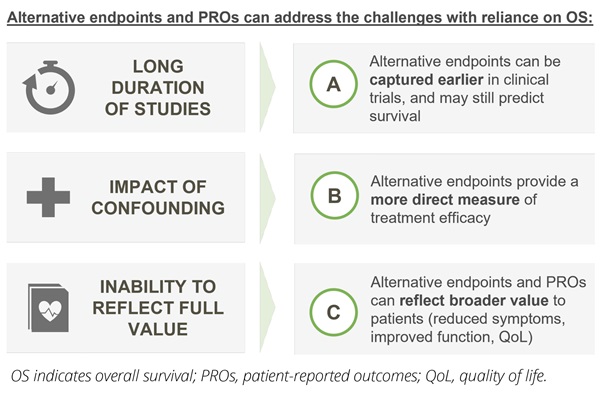
Firstly, in settings where time to demonstrate OS is extensive, endpoints such as time to disease progression or improved patient response provide the opportunity to predict survival outcomes in a time frame that is more meaningful to patients. Across tumor types, the use of PFS as a primary endpoint has been associated with a time savings of approximately 12 months, while overall response rate has been associated with a time savings of approximately 18 months.14 In well-managed and curative settings, time savings can be much greater. For example, the measurement of pCR in the neoadjuvant setting in early breast cancer can be captured on a timescale of months, rather than decades.15
Secondly, in early stage disease, use of alternative endpoints provides the opportunity for a more direct measure of the efficacy of a treatment. In cancer clinical trials, at the point of disease progression, a number of options are available: the patient can cross over to the treatment arm of the trial, the patient can switch to a variety of different treatments outside of the initial clinical trial, the patient can continue with the same treatment for symptomatic benefit, or the patient can receive no additional therapy. The variety of treatment options following progression can obscure the effect of the initial therapy on OS.16 Endpoints that are measured at or before progression (including PFS, DFS, and pCR) are measured upstream of the event that divides patients into different subgroups and can therefore provide a more reliable indication of the clinical benefit of the drug of interest.17
While alternative endpoints that include death by any cause (such as PFS and DFS) can still be difficult to interpret in the face of noncancer-related deaths, they are easier to interpret than OS because progression and recurrence events will occur first for many patients prior to death. Endpoints that do not measure death at all, such as pCR, are not susceptible.
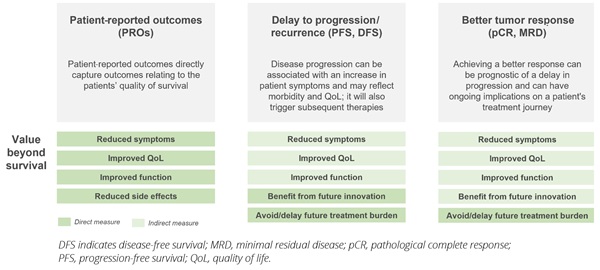
Finally, alternative endpoints, with inclusion of PRO measures, provide the opportunity to reflect the quality of patient survival, enabling a more holistic assessment of the value of new therapies. PROs capture the impact of a novel therapy on the physical, emotional, social, and psychological functioning from the patient perspective, and there is growing cross-stakeholder interest in the use of these outcomes in HTAs as complementary to objective clinical measures.18 In addition, there is increasing recognition of the value of endpoints such as PFS, DFS, and pCR in providing additional insights into the quality of a patient’s life.19 These endpoints can capture a treatment’s impact on lowering the burden of disease, which can be associated with a reduction in cancer-related symptoms, improved patient functioning, and increased QoL.20 They also reflect important implications for the patient’s ongoing treatment journey and the burden of treatment itself—achieving complete response in the neoadjuvant or adjuvant setting may, for example, give patients the opportunity to stop treatment or avoid surgery.
The stand-alone value of alternative endpoints varies by setting. In incurable settings such as multiple myeloma, where patients will cycle through multiple lines of therapy, it has been shown that the extent and duration of patient response decreases with successive lines of therapy, while the burden of symptoms and the toxicity of treatment to the patient increases.21 Delaying time to progression for as long as possible in early lines of therapy is therefore a key goal in its own right for physicians and for patients to reduce the burden of disease and the burden of treatment to the patient for as long as possible. In curative settings, preventing disease recurrence is a highly valued outcome, not just for its prediction of survival but to avoid the return of symptoms, the significant psychological impact, and the burden of further treatments.19,20 Complete response to therapy in the neoadjuvant setting can also be a key goal given the ongoing treatment implications for the patient. In early breast cancer, for example, achieving pCR in the neoadjuvant setting enables patients to undergo less invasive surgery and avoid additional lines of chemotherapy prior to surgery. In a study on patient preferences, pCR was found to be the most important outcome to patients—above DFS, OS, and occurrence of side effects.22
A final factor that should be taken into consideration by regulatory, reimbursement, and clinician decision makers is the opportunity to benefit from future innovation if disease progression is slowed. Given the rapid rate of scientific advances across indications, delaying the onset of metastatic and advanced cancer can provide more patients the chance to benefit from novel therapies and potential cures that are currently in development (Figure 3).
Figure 3. While payers recognize the relevance of a broader set of outcomes, mortality is still prioritized (responses from former payers in the United Kingdom, Germany, France, Italy, Japan, and Canada; research conducted in October 2022)
Ensuring future HTAs result in the best outcomes for patients: actions to support greater use of alternative endpoints and PROs in decision making
While payers acknowledge the limitations associated with reliance on OS benefit and are open to increasing the use of alternative endpoints and PROs in assessments, there are a number of barriers to greater adoption of these endpoints in HTA decision making. Given the potential benefits of increasing the use of alternative endpoints and PROs as either predictors of survival and/or measures of stand-alone value, there is a need for all stakeholders to work together to address current concerns and ensure these endpoints are more fit-for-purpose for use in decision making.
Actions to maximize the potential of alternative endpoints as surrogates for survival
The primary concern preventing greater use of alternative endpoints as predictors of survival is a lack of confidence that these endpoints translate into survival outcomes. And the stakes are high. If the endpoint is a poor predictor of survival, patients may be exposed to potential harm with no additional benefit.
The accepted approach to assessing the level of surrogacy of an endpoint for OS is to conduct meta-analysis of multiple randomized controlled trials in a given tumor type, and to quantify the level of correlation between the effect of treatment on the surrogate and on survival. However, there remains a lack of consensus among regulators and payers on what the acceptable threshold of correlation should be, the number of randomized controlled trials that must be analyzed, and the required specificity of the analysis to line of therapy, tumor type, and class of drug.23,24 This absence of stakeholder consensus results in inconsistent assessments of surrogacy, inconsistent regulatory and reimbursement decision making, and greater mistrust in the use of endpoints as surrogates.23–25
In addition, there is increasing understanding across stakeholder groups that while meta-analysis of randomized controlled trials is considered the best approach to assess surrogacy, demonstrating a high level of surrogacy is not always possible. Indeed, despite substantial effort, high levels of correlation between alternative endpoints and OS have rarely been detected.25 In some cases, this may be driven by a lack of underlying relationship between the surrogate and OS. However, it is important to recognize that there are often other factors at play. The effect of confounding from subsequent lines of therapy and impact of competing noncancer-related deaths will have an impact on OS data but not on the surrogate, and this can obscure true correlation between the two. In addition, an insufficient difference between control and treatment arms, small trial numbers for meta-analyses, lack of consistency in trial design and treatment approaches (eg, different dose, different combinations, different target populations), inconsistent definitions of alternative endpoints, and/or inconsistent measurements of the surrogate across trials can reduce the quality of the data obtained and can make it harder to detect statistically significant correlations.6 Furthermore, if the underlying correlation varies with the subset of tumor type or class of therapy, meta-analyses that are too broad may mask a correlation that exists within these groups, as there often are not sufficient trial data with the required specificity. Considering the benefits of using alternative endpoints to shorten clinical trial durations where correlation to OS does exist, there is a need to reach greater cross-stakeholder consensus on how and when to use endpoints as surrogates in the absence of definitive evidence for surrogacy and to create frameworks to deal with the often unavoidable uncertainty.
To be successful, both approaches require cross-stakeholder action. Ensuring open access to trial data and consistent measurement of endpoints across trials can accelerate the ability to conduct validation studies. Collaboration between regulators, payers, academics, and industry will be required to support the development of more practical and transparent assessments of surrogacy. A UK-based health economist interviewed during research for this paper elaborates on this:
“ A key concern for payers is the lack of evidence to support surrogacy, but looking for complete surrogacy is setting the bar very high. We just want to know that these measures are reasonable predictions of the longer-term outcomes. There is also a difference between not knowing for sure if something is a surrogate because there aren’t sufficient data and data that show a definitive lack of surrogacy.”
— Health Economist
Patients and clinicians can raise awareness of the need for surrogates in the absence of mature OS data to enable timely access to novel therapies and to encourage other stakeholder groups to find practical solutions. And industry commitment to continued monitoring of long-term outcomes in combination with conditional access agreements can be leveraged to accelerate access while sharing the increased risk of uncertainty.
Actions to enable greater use of alternative endpoints and PROs
The first barrier to greater use of alternative endpoints and PROs as measures of stand-alone value in reimbursement decision making is the relative weighting currently given to different outcomes. While there is growing acknowledgment by clinicians, regulators, and health economists that mortality may not always be the most important outcome to the patient, reimbursement decision making continues to prioritize survival. For example, EUNetHTA 21 guidelines on the selection and assessment of outcomes state that while morbidity and QoL impact are valued, these are not viewed as final outcomes and are considered lower than mortality in the hierarchy of outcomes.26
The gap between the perspectives of patients/clinicians and payers regarding the relative importance of mortality compared to other value drivers across indications may be resulting in assessments that do not give sufficient weighting to outcomes that matter most to patients nor reflect how these priorities change depending on different cancer types and stage (eg, early breast cancer versus multiple myeloma). Data from a targeted research project involving 11 former payers from 6 markets (ie, United Kingdom, Germany, France, Italy, Japan, and Canada) highlighted that while payers recognize the relevance of a broader set of outcomes, mortality is still prioritized (Figure 4).
Figure 4. Payer perceptions on relevance of value drivers in early breast cancer

In the absence of mature OS data, decision making should give sufficient weighting to outcomes that matter most to patients and patient access should not be denied. There are a number of cross-stakeholder actions that can be taken to ensure this:
• The patient community can raise awareness of priorities within a disease area and conduct studies to compare patient and payer value drivers to better demonstrate the disconnect between stakeholder groups
• Industry, clinicians, and academics can conduct studies to quantify the clinical and economic benefits to society of improving the quality of patient survival
• Early engagement from regulators and payers on the type of evidence required and commitment to evidence review is essential given the likely investment required for these studies
In addition to ensuring that outcomes related to the quality of survival are given sufficient weighting in reimbursement decision making, there is a need to ensure that the alternative endpoints and PROs used in clinical trials are suitable measures of these outcomes.
Improve the PROs toolkit and PROs data collection methodologies
The use of PROs to provide a direct measure of the quality of patient survival is already accepted in many HTAs. A recent review of the extent to which PROs and QoL data influence the recommendations made by HTA agencies in Germany, France, the United Kingdom, and Scotland found that the submission of PROs data is valued by HTA agencies.27 Demonstration of improvement in QoL led to higher benefit ratings by the Federal Joint Commission (G-BA) and Haute Autorité de santé (HAS), and supported clinical benefit assigned by Scottish Medicines Consortium (SMC) and the National Institute for Health and Care Excellence (NICE).27 Furthermore, in a number of cases, strong PRO data led to a positive recommendation despite a lack of OS data.27 As such, acceptance of these endpoints in and of themselves does not present a key barrier to greater use in decision making.
However, generating meaningful PRO data in a clinical trial can be challenging, which in turn limits the ability to use PROs effectively in reimbursement decision making. Well-established instruments such as the EQ-5D and EORTC QLQ-C30 are well understood by payers, and, because they are generic, support comparisons of the level of benefit across indications. However, these instruments are not specific to the type or stage of disease and concerns have been raised by clinicians, patients, and academics that they are not sensitive enough to provide clinically meaningful insights into the outcomes that are most relevant to a specific patient population. In addition, PRO data in clinical trials often have high rates of missing data.28,29 This limits the ability to use the results in decision making and can skew findings toward patient groups who are more cooperative in filling out the forms but are not representative of the broader patient population. There is a need for more focused and flexible approaches—by measuring symptoms such as fatigue, treatment symptoms, and physical function—to improve analysis and interpretation of PROs to support decision-making processes.
A number of steps can be taken across all stakeholder groups to update and improve the PROs/QoL toolset, reduce the rates of missing data, and provide clear understanding of the patient experience of treatment to ensure future studies are better able to capture the full value of a drug to the patient:
• Academics, clinician, and patient communities need to collaborate to define more disease- and concept-specific questionnaires (eg, fatigue), as well as develop instruments that better reflect patient priorities—both of which will build credibility with decision makers around the validity of PROs
• Payers and regulators will be essential to ensure that novel PRO tools developed are fit-for-purpose and enable comparisons between treatments and cancers similar to OS
• Patient advocacy groups, clinicians, and industry should educate the patient community around the importance of compliance to improve data-collection methodologies and minimize missing data (eg, through flexible and patient-centered approaches to patient data collection, such as online questionnaires)
Raise awareness and generate evidence for the stand-alone value of alternative endpoints
The key concern preventing greater use of alternative endpoints as measures of stand-alone value is a lack of confidence in the long-term benefit to the patient. There is already recognition by payers that delaying progression and extending disease-free periods have value to the patient, but these outcomes are not being given as much weight in HTAs as direct measurement of functional or QoL outcomes.
Unlike regulators who can make positive risk-benefit decisions based on an understanding that lower levels of disease are valuable for the patient, payers need to quantify this benefit and assess and compare the long-term impact of drugs. Most of the rigorous evidence generation for endpoints that measure delay to progression or tumor response has been focused on demonstrating surrogacy for survival, rather than understanding and quantifying the long-term benefit of these endpoints to the quality of survival in different indications.
“What we have at the moment is a lack of data that these endpoints reflect patient benefit beyond survival, without surrogacy.”
—Regulator (former European Medicines Agency expert)
“We as a community have to understand better, and raise awareness of the relevance of achieving, for example, complete response, and the impact this has on the patient’s disease pathway. We have to explain better why it is important to achieve a pCR and we need to support that with evidence.”
— Clinician (oncologist with expertise in early stage breast cancer)
Furthermore, in some diseases and/or for some patients, progression events (determined by laboratory measurements of tumor size) may not always be associated with a functional impact for the patient or the treatments responsible for prolonging time to progression may result in increased toxicities that remove the benefits on QoL.30,31 In addition, the benefit of better disease control may be a short-term rather than a long-term outcome if the patient goes on to relapse. The heterogeneity in the stand-alone value of endpoints beyond OS across indications can lead to a lack of confidence in their use, even in settings where delay to progression or tumor response does reflect significant benefit to patients. This in turn can contribute to inconsistent assessments of the value of these endpoints in reimbursement decision making.
Every stakeholder group can play a key role in ensuring the opportunity to use endpoints beyond OS as stand-alone measures of value in HTA decision making is maximized. For example:
• The patient and clinical community need to educate other stakeholders on the clinical importance of delaying progression or improving tumor response within a given indication.
• For industry, investing in studies to better quantify the stand-alone value of endpoints that measure the level of benefit for patients will be important, either by formally linking these endpoints to health-related-QoL measures, or through collaboration with health economics and outcomes research experts to assess the long-term clinical and economic benefits of better disease control.
• Given the associated costs of evidence generation, upfront engagement with regulators and payers, and buy-in to proposed studies, will be required.
Conclusions
Continued reliance on demonstrating an OS benefit as the primary measure of innovation in oncology presents key limitations that restrict patient access to innovative therapies and leads to higher healthcare costs. As cancer treatments improve, the limitations associated with OS will continue to grow. Alternative endpoints and PROs provide the opportunity to capture the full value of novel therapies in a timely manner. However, concerns over the ability of alternative endpoints and PROs to reflect long-term benefit to patients are limiting their use in reimbursement decision making.
Continued progress in oncology depends on all stakeholders working together to overcome these barriers and ensure reimbursement decision making can result in the best outcomes for patients. Patients can raise awareness and educate other stakeholders on the value of alternative endpoints and PROs, while other stakeholders can educate patients on the importance of compliance to minimize missing data and improve the PRO/QoL toolset. Furthermore, industry, clinicians, and academics can conduct various studies that help to quantify the benefits of alternative endpoints and PROs, while regulators and payers can invest and/or engage in these to build momentum.
Conflict of Interest Disclosures: All authors are employees and shareholders in GSK. Dr Cimen is also a shareholder in AstraZeneca.
Funding/Support: This work was funded by GSK. LEK Consulting provided support in the development of this article.
References
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. Accessed December 7, 2022. https://gco.iarc.fr/today
2. Nayak M, George A, Vidyasagar M, et al. Quality of life among cancer patients. Indian J Palliat Care. 2017;23(4):445. doi:10.4103/IJPC.IJPC_82_17
3. van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070-1090.e9. doi:10.1016/j.jpainsymman.2015.12.340
4. NHS inform. Cancer-related fatigue. Accessed June 28, 2023. https://www.nhsinform.scot/illnesses-and-conditions/cancer/side-effects/cancer-related-fatigue#:~:text=Cancer%2Drelated%20fatigue%20(CRF),to%20keep%20a%20fatigue%20diary
5. Nugusse T, Lemlem SB, Deressa J, Kisa S. Prevalence of fatigue and associated factors among cancer patients attending Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Cancer Manag Res. 2021;13:1909-1916. doi:10.2147/CMAR.S291298
6. Lux MP, Ciani O, Dunlop WC, Ferris A, Friedlander M. The impasse on overall survival in oncology reimbursement decision-making: how can we resolve this? Cancer Manag Res. 2021;13:8457-8471. doi:10.2147/CMAR.S328058
7. Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based study. Lancet. 2015;385(9974):1206-1218. doi:10.1016/S0140-6736(14)61396-9
8. Tufts Center for Study of Drug Development. Tufts CSDD Impact Report May/Jun 2021. Tufts University. 2021
9. Capocaccia R, Gatta G, Dal Maso L. Life expectancy of colon, breast, and testicular cancer patients: an analysis of US-SEER population-based data. Ann Oncol. 2015;26(6):1263-1268. doi:10.1093/annonc/mdv131
10. Xu Y, Liu H, Cao Q, Ji J, Dong R, Xu D. Evaluating overall survival and competing risks of survival in patients with early-stage breast cancer using a comprehensive nomogram. Cancer Med. 2020;9(12):4095-4106.doi:10.1002/cam4.3030
11. Saad ED, Buyse M. Statistical controversies in clinical research: end points other than overall survival are vital for regulatory approval of anticancer agents. Ann Oncol. 2016;27(3):373-378. doi:10.1093/annonc/mdv562
12. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968
13. Falcone R, Lombardi P, Filetti M, et al. Oncologic drugs approval in Europe for solid tumors: overview of the last 6 years. Cancers (Basel). 2022;14(4):889. doi:10.3390/cancers14040889
14. Chen EY, Joshi SK, Tran A, Prasad V. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern Med. 2019;179(5):642. doi:10.1001/jamainternmed.2018.8351
15. FDA. Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval | FDA. Accessed June 28, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathological-complete-response-neoadjuvant-treatment-high-risk-earlystage-breast-cancer-use
16. Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642-1649. doi:10.1093/jnci/djp369
17. Skelly A, Dettori J, Brodt E. Assessing bias: the importance of considering confounding. Evid Based Spine Care J. 2012;3(01):9-12. doi:10.1055/s-0031-1298595
18. Wilson R. Patient led PROMs must take centre stage in cancer research. Res Involv Engagem. 2018;4(1):7. doi:10.1186/s40900-018-0092-4
19. Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. Am J Cancer Res. 2022;11(4):1121.
20. Harbeck N, Schneeweiss A, Thuss-Patience P, et al. Neoadjuvant and adjuvant end-points in health technology assessment in oncology. Eur J Cancer. 2021;147:40-50. doi:10.1016/j.ejca.2021.01.006
21. Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. 2016;175(2):252-264. doi:10.1111/bjh.14213
22. Thill M, Pisa G, Isbary G. Targets for neoadjuvant therapy – the preferences of patients with early breast cancer. Geburtshilfe Frauenheilkd. 2016;76(05):551-556. doi:10.1055/s-0042-101025
23. Ciani O, Grigore B, Blommestein H, et al. Validity of surrogate endpoints and their impact on coverage recommendations: a retrospective analysis across international health technology assessment agencies. Med Decis Making. 2021;41(4):439-452. doi:10.1177/0272989X21994553
24. Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017;15(1):134. doi:10.1186/s12916-017-0902-9
25. Gyawali B, Hey SP, Kesselheim AS. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine. 2020;21:100332. doi:10.1016/j.eclinm.2020.10033226.
26. EUnetHTA. Individual Practical Guideline Document. Accessed June 28, 2023. https://www.eunethta.eu/wp-content/uploads/2022/12/EUnetHTA21-D4.5-Practical-Guideline-on-Applicability-of-Evidencev1.0.pdf
27. Hintzen C, Lie X, van Engen A, New M. PROS in oncology HTA decisions, do they matter? Value Health. 2017;20(9):A470-A471. doi:10.1016/j.jval.2017.08.410
28. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353-367. doi:10.2147/PROM.S156279
29. Mercieca-Bebber R, Palmer MJ, Brundage M, Calvert M, Stockler MR, King MT. Design, implementation and reporting strategies to reduce the instance and impact of missing patient-reported outcome (PRO) data: a systematic review. BMJ Open. 2016;6(6):e010938. doi:10.1136/bmjopen-2015-010938
30. Hwang TJ, Gyawali B. Association between progression-free survival and patients’ quality of life in cancer clinical trials. Int J Cancer. 2019;144(7):1746-1751. doi:10.1002/IJC.31957
31. Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol. 2012;30(10):1030-1033. Doi:10.1200/JCO.2011.38.7571

