Opening Up a Discussion on Biosimilar Value Assessment
Teresa Barcina Lacosta, MSc, PharmD, KU Leuven, Leuven, Belgium; Alex Gee, Senior Director, Pricing and Market Access, Parexel International, London, England, United Kingdom; András Inotai, PhD, Center for Health Technology Assessment, Semmelweis University, Budapest, Hungary; Catarina Lopes Pereira, MSc, Chair, ISPOR Biosimilar Special Interest Group, Brussels, Belgium; Steven Simoens, PhD, KU Leuven, Leuven, Belgium
Background
A few months ago, the ISPOR Biosimilar Special Interest Group (SIG) had the pleasure of organizing a forum on biosimilar value assessment at the ISPOR Europe Conference in Vienna. It was an ideal opportunity to present key learnings from our research project and to open up a conversation with the conference audience on knowledge gaps and challenges that relate to assessing biosimilars value. It was central to this session to discuss what has been and what is the foreseen role of health technology assessment (HTA) for biosimilars, and to question whether biosimilars value assessment should be restricted to a price comparison between the reference biologic and the biosimilars. The main highlights are summarized in the sections below.
A trend toward streamlining biosimilars value assessment: key considerations
According to our SIG’s research investigating the role of HTAs for biosimilars, there has been a general aim to streamline biosimilar value assessment, mainly to make biosimilars more readily available for patients and to decrease the workload that HTA institutions are subjected to. Simplifying value assessment processes for biosimilars has generally implied circumventing formal HTA submissions and, when possible, basing reimbursement decisions on price comparisons.
Although it can be convenient to streamline the assessment of biosimilars, it is also necessary to determine which cases would require more detailed assessments. In fact, our research tells us that some circumstances may require conducting economic evaluations for new biosimilar entrants at the HTA level. For instance: (1) when the originator has not been approved for reimbursement before having to appraise new biosimilar entrants in a specific country; and (2) when biosimilars have different formulations and administration routes with respect to the originator. In the first case, the nonreimbursed originator will not likely qualify as a policy-relevant comparator for the new biosimilar entrant, and comparators with different active molecules and potentially different cost-effectiveness profiles may be selected. In this context, a proper comparative assessment of these technologies would require a full economic evaluation. In the latter case, relying on a simple price comparison for products with different formulations or administration routes may not provide a full picture of the real-world value of biosimilars.
"There has been a general aim to streamline biosimilar value assessment, mainly to make biosimilars more readily available for patients and to decrease the workload that HTA institutions are subjected to."
Beyond price comparisons
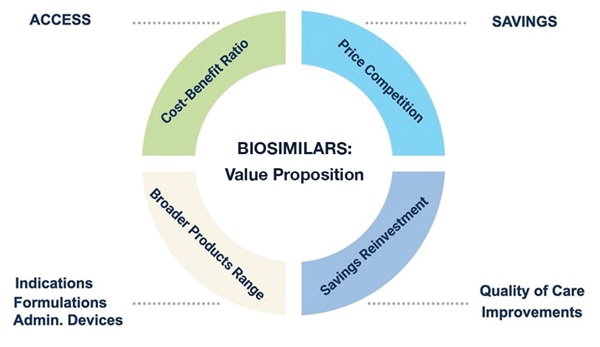
Our research suggests that applying HTA methodologies to biosimilars can generate additional evidence on the value proposition of these products (Figure). So far, the lack of an established HTA pathway for biosimilars in certain jurisdictions has limited the potential to valorize broader elements of value, such as the provision of value-added services, extending the offer of marketed formulations and devices, and supporting supply-chain reliability. During the session, members of the audience expressed their interest in valorizing these aspects, especially when it comes to the differential provision of value-added services by sponsors.
However, in practice, several challenges relate to going beyond basing reimbursement decisions for biosimilars on price comparisons. Some of the biggest challenges are the limitations in data availability. From the perspective of lower income countries, if value assessment goes beyond price comparisons, it may be hindered by limited available data or—in the case of emerging countries—limited HTA capacities. When reflecting on potential claims of added value submitted by biosimilar sponsors for reimbursement, biosimilars are often launched with the minimal data package to support regulatory approval. If there is then the desire to claim a price premium (say for example, on the basis of ease of administration), then there needs to be an appropriate evidence package to support that. So, a challenge is making sure that data are available to make a robust decision.
In view of these challenges, it is still in question whether it would be feasible to account for these broader elements of value at the HTA level. In accordance with the mission of our SIG, which is to discuss emerging issues of biosimilars focusing on health economics and outcomes research and reimbursement policy, we will continue to monitor new developments in biosimilar value assessment and to investigate solutions to current challenges.
Figure.


Explore Related HEOR by Topic

