Early Experience With Health Technology Assessments for COVID-19 Treatments
Xenia F. Sitavu-Radu, PhD, MSc, IQVIA, London, England, United Kingdom; Tulika Paul, MTech, IQVIA, Gurugram, Haryana, India; Jennifer G. Gaultney, PhD, MPH, IQVIA, London, England, United Kingdom
Background
Over the past decade, the global healthcare market has seen an increase in the launch of health technologies.1 New health technologies are approved daily, which may be attributed to aging population, rising income levels, emerging medical conditions, and advancements in knowledge and technology.2,3 To assess how emerging therapeutic innovations perform compared to existing care, a standard policy tool is required to evaluate evidence across all dimensions.4 Health technology assessment (HTA) is a multidisciplinary tool that systematically reviews the clinical, social, economic, organizational, and ethical evidence of a health technology to determine its value compared to standard of care. The valuation of the technology informs policy decision making, including reimbursement and pricing decisions, that facilitates access to healthcare innovations. Thus, HTA contributes to the maintenance of an equitable, efficient, and high-quality healthcare system.4
Emergence of COVID-19 pandemic severely impacted the healthcare sector with potential long-term impact on the conduct and operation of healthcare systems and technologies.5 Examples of such impact included delay or disruption in the conduct of clinical trials, regulatory reviews, and inspections and audits of clinical trial sites as a result of restrictions imposed by travel bans, hospital/clinic visits, and social distancing precautions during the pandemic.6,7 This research was conducted to understand the pandemic’s impact on HTA and payer communities.
Methodology
A total of 15 HTA agencies, covering Europe, Canada, and Australia, were selected based on availability of assessment reports for evaluations undertaken between January 2020 and April 2022. Based on the completeness of information available, 9 of the 15 HTA agencies were retained for this research: Canadian Agency for Drugs and Technologies in Health (CADTH, Canada), Finnish Medicines Agency (FIMEA, Finland), Federal Joint Committee (G-BA, Germany), French National Authority for Health (HAS, France), Belgian Healthcare Knowledge Centre (KCE, Belgium), National Institute for Health and Care excellence (NICE, England), Scottish Medicines Consortium (SMC, Scotland), Dental and Pharmaceutical Benefits Agency (TLV, Sweden) and National Health Care Institute (ZIN, Netherlands).
"Only 1 appraisal (remdesivir submission to TLV) included a cost-effectiveness model. Budget impact models were included in 3 appraisals of COVID-19 technologies."
Relevant data to support our analysis for the selected agencies were extracted from the HTA Accelerator™ (HTAA), which is a comprehensive database that includes publicly available HTA reports. For the purpose of our research, we focused on the following information: assessment type, clinical evidence evaluated, economic model evaluated, drivers of cost-effectiveness, budget impact analysis performed, recommendation, and rationale.8 A gray literature search was also conducted to identify any formal communication from HTA bodies describing their approach to prioritizing as well as any adaptation to processes for the appraisal of COVID-19 interventions.
Results
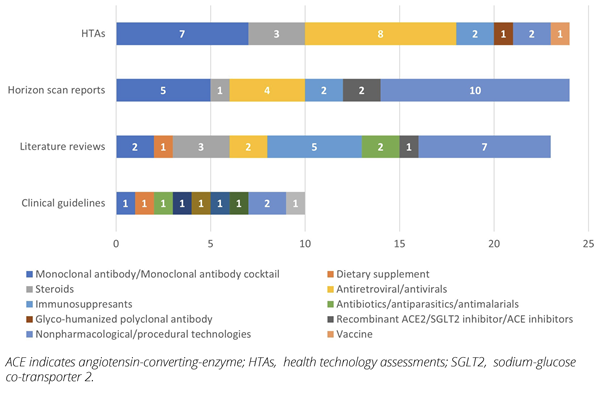
Overall, approximately 3200 evaluations were undertaken by the 9 HTA agencies selected between 2020 to 2022, of which a total of 91 evaluations (2.84%) were undertaken for various COVID-19 technologies for prevention, testing, and treatment. CADTH (n=36), NICE (n=22), and ZIN (n=13) conducted the most evaluations, while FIMEA, G-BA, and TLV undertook 1 evaluation each. No SMC and KCE evaluations of COVID-19 technologies were identified (Figure 1A). The majority of the evaluations consisted of horizon scanning reports (28.6%), literature reviews of available evidence (27.5%), and clinical guidelines (17.6%) undertaken by HTA agencies; the evaluations assessed less innovative technologies approved in other indications that have been reassessed for COVID-19 treatment (Figure 2). Over the study period, only 24 HTAs were identified, with most appraisals for antivirals (33.3%) and monoclonal antibodies (29.2%) (Figure 1B).
Figure 1: Number of published evaluations by HTA agency: (A) Guidance and guidelines issued by HTA agencies (B) HTAs
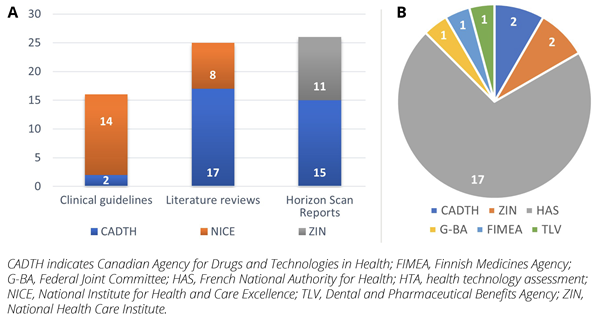
Figure 2: COVID-19 technologies by evaluation type
Clinical guidelines and guidance issued by HTA agencies
Guidelines and guidance issued by HTA agencies were published as horizon scanning reports (n=26), literature reviews (n=25), and clinical guidelines (n=16) by CADTH (n=34, 51%), NICE (n=22, 33%) and ZIN (n=11, 16%) (Figure 1A). Horizon scanning reports primarily focused on COVID-19 treatments (53.8%), such as monoclonal antibodies (casirivimab/imdevimab, regdanvimab, sotrovimab, bamlanivimab/etesevimab) and antivirals (remdesivir, favipiravir), while a few were for preventive or diagnostic procedures, testing kits, vaccines, wearable devices, and long-term post-COVID-19 conditions. Similarly, most literature reviews (64%) focused on treatments such as interleukin-6 antagonists (sarilumab, tocilizumab, anakinra) and steroids, while the rest were summarizing evidence on testing kits and procedures. The majority of the clinical guidelines (56.3%) were evaluated by NICE as rapid guidelines on potential treatments for COVID-19 (steroids, immunosuppressants, antibiotics, antimalarials, antivirals, vitamin D). Additionally, few other evaluations were conducted for COVID-19–related complications, respectively for cardiovascular (n=2), digestive (n=1), respiratory (n=2), and blood and immune disorders (n=2) assessed by CADTH, NICE, and ZIN.
None of the horizon scan reports, literature reviews, and clinical guidelines published in 2020 and 2021 provided recommendations; reasons for not making recommendations included limited evidence resulting in uncertain clinical benefits, limited generalizability to local clinical settings, and uncertain budget impact. In April 2022,a only 1 clinical guideline that reviewed pharmacological therapies for COVID-19 provided a positive recommendation with restriction from CADTH.b
HTAs of COVID-19 treatments
Of the 24 HTAs on COVID-19 treatments undertaken between 2020 and 2022,16 appraisals were submitted to HAS (67%), and 2 were submitted to CADTH (8%), while 1 each was submitted to FIMEA (4%), G-BA (4%), and TLV (4%) (Figure 1B). Additionally, 1 appraisal for medical device was submitted to HAS and 2 procedure appraisals to ZIN. A total of 13 HTAs received positive recommendation, 6 received positive recommendation with restrictions, and 3 received negative recommendation, while 2 received no recommendation (Figure 3A).
Figure 3: (A) Recommendations provided for HTAs; (B) Rationale for positive recommendations by HTA agency
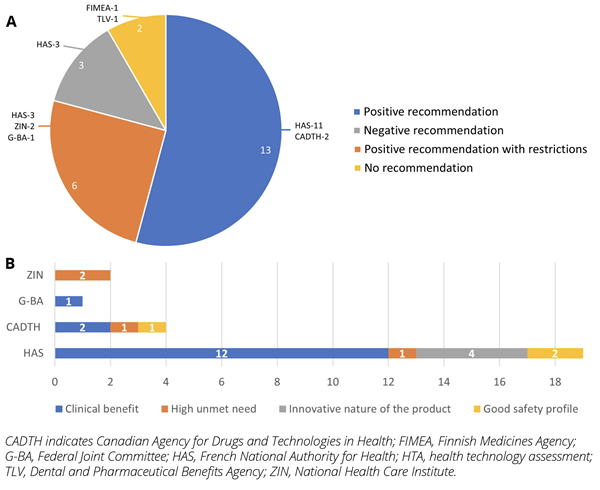
Remdesivir was one of the most commonly evaluated treatments (16.7%); only HAS and G-BA recommended remdesivir but restricted its use to a subgroup of patients,c while FIMEA and TLV did not provide a recommendation for remdesivir. FIMEA and TLV noted that remdesivir shortened recovery time and potentially reduced mortality versus placebo and can be considered cost neutral if it shortens hospitalization by an average of 3 days. Tixagevimab/cilgavimab (pre-exposure prophylaxis), casirivimab/imdevimab (post-exposure prophylaxis and treatment for COVID-19), and sotrovimab (treatment for COVID-19) received positive recommendation based on clinical benefit and innovative nature of the products. Nirmatrevir/ritonavir for treatment of mild-to-moderate COVID-19 was recommended based on clinical benefit, good safety profile, and unmet need. Tocilizumab was recommended for treatment of COVID-19 based on clinical benefit but restricted to adult COVID-19 patients who require oxygen supplementation and had received prior systemic corticosteroid therapy (Figure 3B). Molnupiravir, XAV-19, and anakira received a negative recommendation from HAS based on uncertain clinical benefit.
Evidence included in COVID-19 evaluations
The clinical guidelines and guidance issued by HTA agencies were primarily informed by systematic reviews (n=29); other sources of evidence included randomized controlled trials (n=18), observation/cohort data (n=16), meta-analyses (n=5), and expert opinion (n=1). Few evaluations included budget impact models, undertaken by CADTH (n=12) and ZIN (n=7) (Figure 4A).
Figure 4: Type of clinical and economic evidence used by each HTA agency: (A) Guidance and guidelines issued by HTA agencies (B) HTAs
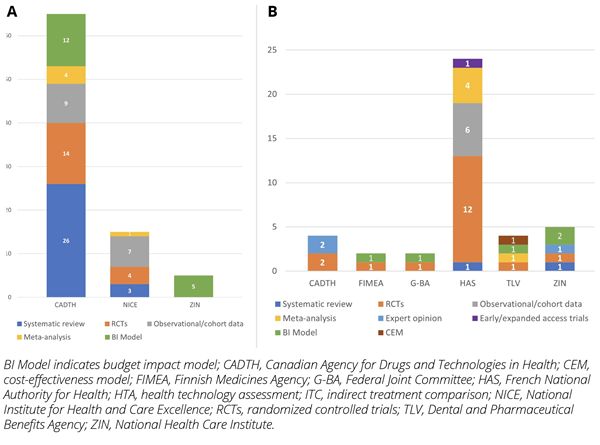
Clinical evidence submitted for HTAs was informed from randomized controlled trials (n=18), observation/cohort data (n=6), meta-analysis (n=5), expert opinion (n=3), early/expanded access trials (n=1), and systematic reviews (n=2). In terms of economic evidence, only 1 appraisal (remdesivir submission to TLV) included a cost-effectiveness model. The drivers of cost-effectiveness were the cost of treatment and associated treatment effect. Budget impact models were included in 3 appraisals of COVID-19 technologies only, to TLV (n=1), FIMEA (n=1), and G-BA (n=1) (Figure 4B).
"Economic evidence was deprioritized in the assessments of COVID-19 interventions, possibly to facilitate patient access. The aftermath of the pandemic highlighted a clear need for early cross-functional stakeholder collaboration."
Prioritization and changes in timelines
Gray literature search revealed that NICE, HAS, and CADTH prioritized COVID-19 interventions as therapeutically critical.9,10 NICE reported delays due to suspension of all appraisals during the first and second quarters of 2020, which led to a 25% drop in HTA publications in 2020 compared to the average in previous years, 2015 to 2019.6 In April 2020, CADTH postponed planned drug review consultations; however, CADTH managed to continue operating with minimal impact on core and additional COVID-19 activities. CADTH, G-BA, and HAS moved to a virtual office to continue delivering programs and services.9,11 SMC had stated that COVID-19 interventions were prioritized; however, no individual appraisals undertaken by SMC were identified as the agency collaborated with NICE and other organizations in the United Kingdom under the program for “Research to access pathway for investigational drugs for COVID-19” (RAPID C-19) to monitor and evaluate emerging trial evidence of potential COVID-19 treatments during the pandemic.12,13
Conclusion
Between 2020 and 2022, approximately 3% of the overall evaluations undertaken by the selected 9 key HTA agencies were of COVID-19 technologies. HTA agencies prioritized COVID-19 and evaluated data available on potential treatments; out of the 91 evaluations identified, 67 were horizon scanning reports, systematic literature reviews, and clinical guidelines issued by HTA agencies. Most of these evaluations considered less robust data than what the HTA community is accustomed to, with only 18 evaluations including randomized controlled trial evidence; however, almost exclusively no recommendations were made in the January 2020 to April 2022 period due to lack of sufficient data.
On the contrary, the majority of the HTAs submitted (~80%) received a positive recommendation (with/without restrictions), although a very limited number of the submissions included an economic assessment which is a strong evidence requirement of all HTA agencies (1 cost-effectiveness model and 3 budget impact models only were submitted). Clinical evidence submitted was of good quality, with 18/24 submissions including randomized controlled trial data that potentially led to positive recommendations as the majority of positive recommendations were made due to demonstrated clinical benefit (79%), followed by high unmet need and innovativeness of technology (21%).
With the health crisis brought in by the pandemic, research and development activities for testing, prophylaxis, and treatment of COVID-19 were prioritized by manufacturers, regulatory, and HTA agencies, leading to a majority of submissions being reviewed with less robust evidence. Economic evidence was deprioritized in the assessments of COVID-19 interventions, possibly to facilitate patient access. There was limited evidence available to assess the impact of prioritization and adaptation of COVID-19 health technologies by HTA agencies.
The aftermath of the pandemic highlighted a clear need for early cross-functional stakeholder collaboration and integration of processes for regulatory review and HTA to ensure access to high value innovation.
References
1. Research and Development in the Pharmaceutical Industry. Congressional Budget Office. Published April 2021. Accessed June, 2022. https://www.cbo.gov/publication/57126
2. Global Pharmaceuticals Industry Analysis and Trends 2023. GlobeNewswire. Published January 17, 2020. Accessed June, 2022. https://www.globenewswire.com/news-release/2020/01/17/1972092/0/en/Global-Pharmaceuticals-Industry-Analysis-and-Trends-2023.html
3. Pethe A. The role of technology in evolving pharmaceutical sector. Pharmabiz.com. Published August 18, 2021. Accessed June, 2022. http://pharmabiz.com/ArticleDetails.aspx?aid=141925&sid=9
4. Health technology assessment. World Health Organization. Accessed June, 2022. https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/assessment#:~:text=Health%20technology%20assessment%20(HTA)%20refers,health%20intervention%20or%20health%20technol
5. Understanding the hidden costs of COVID-19’s potential impact on US healthcare. Published September 4, 2020. Accessed June, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/understanding-the-hidden-costs-of-covid-19s-potential-impact-on-us-healthcare
6. Impact of COVID-19 pandemic on HTA. PharmacoEcon Outcomes News. 2020;853(1):2. doi:10.1007/s40274-020-6797-2
7. Halloran L. Assessing the impact of COVID-19 on regulatory interactions, inspections, & audits. Clinical Leader. Published March 19, 2020. Accessed June, 2022. https://www.clinicalleader.com/doc/assessing-the-impact-of-covid-on-regulatory-interactions-inspections-audits-0001
8. HTA Accelerator to support strategic payer decisions and market access. IQVIA. Accessed April, 2022. https://www.iqvia.com/solutions/real-world-evidence/health-economics-and-value/hta-accelerator
9. Thomas M, Bailey S, Craddy P, Foxon G. What impact did covid-19 have on hta bodies and pharmaceutical company pricing and market access activities? Value Health. 2020:S670.
10. Lilliu H. Inbeeo. Blog: Reflections on the initial impact of COVID 19 on global market access activities. Published 2020. Accessed June, 2022. https://inbeeo.com/2020/07/31/impact-of-covid-19-on-market-access-activities/
11. CADTH. COVID-19 Response. Published October 29, 2020. Accessed May, 2022. https://www.cadth.ca/covid-19-response-2020
12. Stephens J, Webb N. COVID-19, evidence-based medicine and standards for HTA. https://source-he.com/covid-19-evidence-based-medicine-and-standards-for-hta/
13. Research to access pathway for investigational drugs for COVID-19 (RAPID C-19). https://www.nice.org.uk/covid-19/rapid-c19
Explore Related HEOR by Topic

