Patient Involvement in Regulatory and Health Technology Assessment Processes: A Call for Enhanced Alignment
Michaela Dinboeck, MIB, Novartis Pharma AG, Basel, Switzerland; Neha Noopur, MBA, Novartis Healthcare Pvt Ltd, Hyderabad, Telangana, India; Annekatrin Krause, PhD, Novartis Pharma AG, Basel, Switzerland; Simarjeet Kaur, MPharm, Novartis Healthcare Pvt Ltd, Hyderabad, Telangana, India
Patient Involvement and Decision Making
Patient-focused drug development (PFDD) and understanding patient knowledge and experience are increasingly becoming instrumental in drug development and associated decision making. Inclusion of patients’ expertise about their lived experience brings a different and complementary perspective, compared to stakeholders who are not affected by the disease. Integrating these unique insights in decision making helps drive the development of innovative medicines, improve health outcomes, and transform healthcare. For an effective delivery of a drug within a healthcare system, patients need to trust in processes that can be achieved with greater patient involvement. Bringing new medicines to patients is dependent on 2 sequential processes: (i) the evaluation for marketing authorization by regulatory bodies, and (ii) the value assessment (eg, health technology assessments [HTAs]) for payers and reimbursement. Due to the variety of scientific requirements on how patient input should be captured, authorities and pharmaceutical companies face uncertainty in drug development decisions.
"For an effective delivery of a drug within a healthcare system, patients need to trust in processes that can be achieved with greater patient involvement."
This overview aims to compare the level of patient involvement in regulatory and HTA processes to understand convergence and divergence of approaches across major countries. This was assessed by 4 parameters: (i) overall governance framework for patient involvement, (ii) patient engagement guidance and guidelines for specific processes, (iii) institutional capacity for patient involvement, and (iv) patient community outreach and communication. Health equity is also an important topic for patients and regulators/HTA bodies, yet not the focus of the research at hand as it differs in its nature from the active involvement of patients in decision making.
Patient Involvement With Regulators in Decision Making
Since the early 1990s, the US Food and Drug Administration (FDA),1 European Medicines Agency (EMA)2 and Therapeutic Goods Administration (TGA, Australia)3 have developed methodologies for patient involvement. In 2010, Health Canada,4 Agenzia Italian a del farmaco (AIFA)5 and Swissmedic (Switzerland)6 joined the efforts and since then other regulators followed. In the countries in scope of this overview, frameworks are developed by most regulators to promote patient involvement systematically and consistently in decision making. (Figure 1)
FDA is developing a series of PFDD guidance documents7 to address how stakeholders can gather patient experience data for drug development and regulatory decision making. It has also released guidance for Patient Preference Information in 2016.8 EMA strongly reinforced patient involvement across regulatory activities through its engagement framework and future evolution of patient experience data in medicines’ development and regulatory decision making as discussed in a workshop in 2022.9 Patients are seen as experts like healthcare professionals and both stakeholders are encouraged to work together (eg, Patients’ and Consumers’ Working Party). TGA and Health Canada have structured guidelines to detail the levels of stakeholder or public engagement respectively. FDA, EMA, Health Canada, Swissmedic, and recently AIFA and TGA have a dedicated staff/office to strengthen capabilities for patient involvement reflecting the principle of equity that allows them to participate in stakeholder dialogue in a meaningful way.
In 2021, Medicines & Healthcare Products Regulatory Agency (MHRA, United Kingdom)10 published a Patient and Public Involvement Strategy 2020-2025 defining the process of how to engage and involve the public/patients and launched a pilot program for patient involvement in drug development. Patient involvement would contribute to drug evaluations under the new Innovative Licensing and Access Pathway and safety signals, creating a benchmark against other regulators’ progress. Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) published guidelines on patient involvement in medicines development and regulations that provide structure to patient input.11,12 In Latin America, ANVISA (Brazil) has been carrying out public consultations on health technologies since 2008.13 Recently, China’s Centre for Drug Evaluation finalized new guidelines requesting sponsors of randomized controlled trials to consider patient needs and experience for their study.14
Figure 1: Timelines for Patient Involvement in Decision Making by Regulators
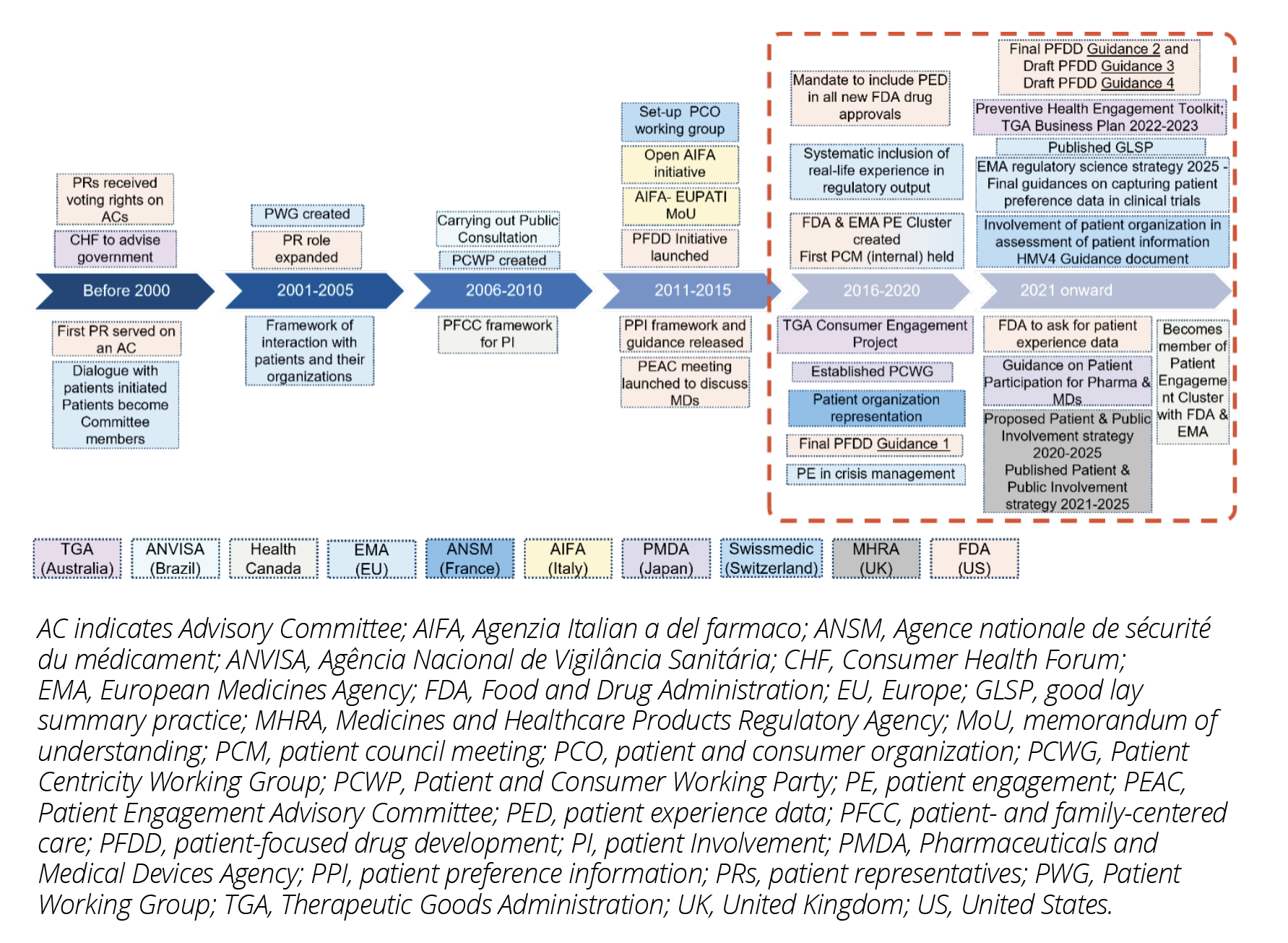
Source: AIFA; BMJ; Clinigma; FDA Patient Engagement; Gov.UK; Health Canada; Partners & networks - Patients and consumers; PMDA; RSP; Swissmedic; TGA.
Patient Involvement in HTA Decision Making
Similar to regulatory bodies, HTA bodies are increasingly incorporating patient involvement into their processes. The Pharmaceutical Benefits Advisory Committee (PBAC, Australia),15 Canadian Agency for Drugs and Technologies in Health (CADTH),16 and National Institute for Health and Care Excellence (NICE, United Kingdom)17,18 are at the forefront with patient involvement through patient community representation in HTA committees almost since 2 decades; Haute Autorité de santé (HAS, France)19 and Red Española de Agencias de Evaluación de Tecnologías Sanitarias (RedETS, Spain)20 began with patient and consumer groups involvement in the past few years (Figure 2). PBAC, CADTH, NICE, Gemeinsamer Bundesausschuss (G-BA, Germany)18,21 and Institute for Clinical and Economic Review (ICER, United States)22 have a well-framed guidance for patients. In addition, these organizations provide capability building resources by creating online libraries (videos, reading materials), or conducting lectures/symposia. They report back on their patient involvement efforts by sharing the meeting outcomes, annual reports, and examples on how the patient/consumer input is used. PBAC Office of HTA consultation hub and HTA Consumer Evidence and Engagement Unit help consumers and patients to be part of HTA processes. Consumer comments are summarized in the meeting minutes and noted in the public summary document for the submission. NICE publishes Public Involvement Programme annual reports with all the patient involvement statistics as well as exit interviews with patients providing feedback.
For European countries, approaches to HTA differ from country to country. Some countries rely on a national process like Germany, while other countries (eg, Italy and Spain) carry out additional processes at a local regional level leading to a fragmentation of methods within a country. This fragmentation might be overcome with the upcoming implementation of the new EU HTA Regulation in 2025,9 including guidance on the involvement of patients and representations as experts and stakeholder from the beginning.
Conitec (Brazil) has been conducting public consultations annually since 2011 and since 2021 has started working directly with patients through testimonials in its committee meetings for all assessed technologies.13 In the United States, there is no nationwide value assessment, and it is fragmented among many stakeholders. One of those is ICER, who initiated patient engagement activities in 2010. In 2020, it launched a new program to augment its efforts to empower and partner with patient organizations to contribute substantively before, during, and even after ICER’s assessments.22
Figure 2: Timelines for Patient Involvement in Decision Making by HTA bodies
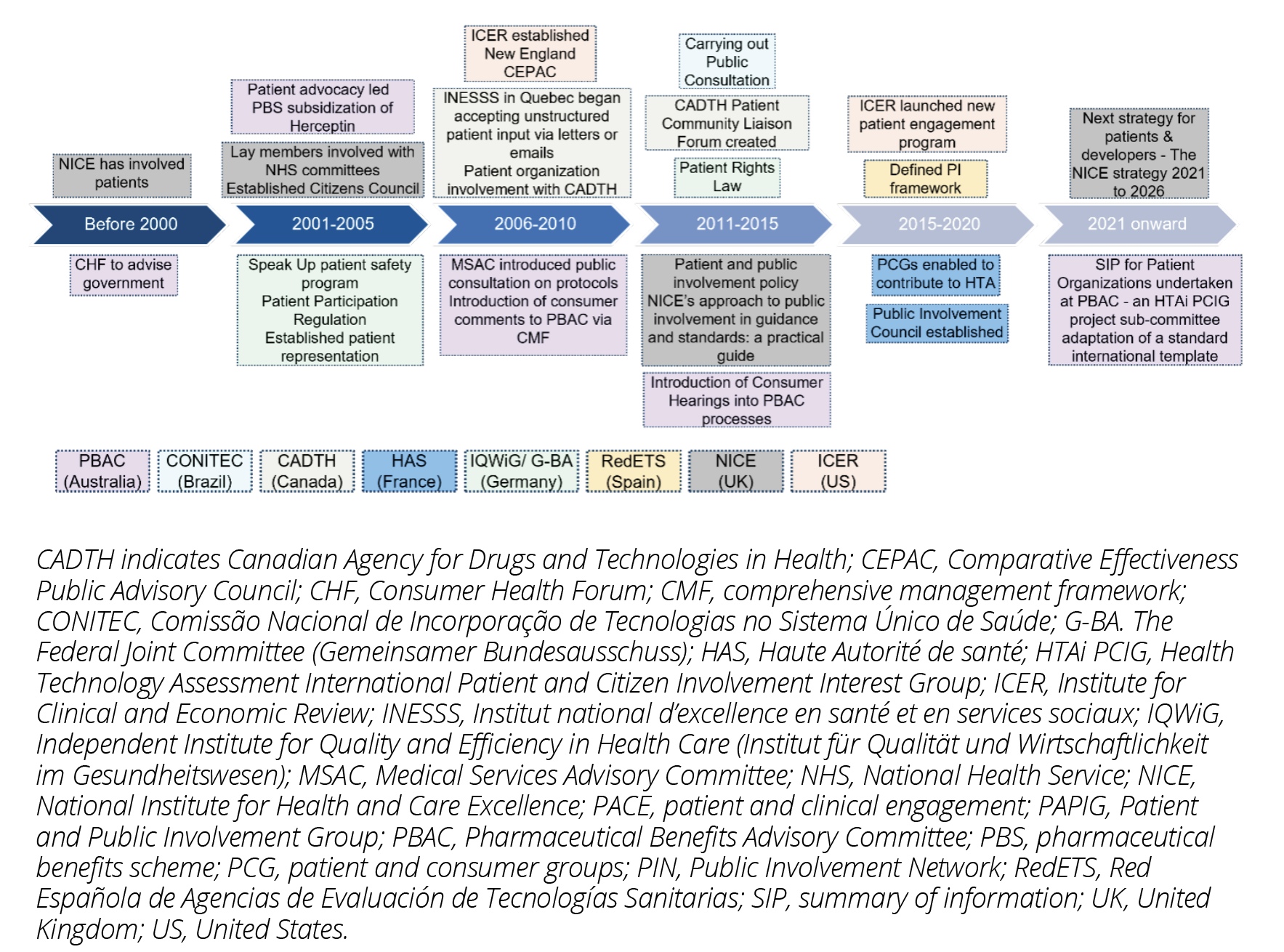
Source: CADTH; Cambridge Core Home; HAS; ICER; IQWiG/G-BA; NICE; PBAC; RSP
As HTA bodies focus more and more on a systematic approach to patient involvement, the act of involving patients in value assessments has long moved away from being a “tick the box” or “nice to have” activity. Patients bring different and complementary perspectives to decision bodies, and their input is critical to value assessment processes. The unique insights coming from patients help the assessors to contextualize the information provided by the sponsor of the study and submissions and to understand whether and how the new technology would or would not help address the issues experienced by patients.23,24
Convergence and Divergence Across Regulators and HTA Bodies Within Countries
Most authorities have included some level of patient involvement in decision making along the medicines’ lifecycle. However, limited published details in English were available for Brazil, China, Italy, Japan, Spain, and Switzerland. Across the United States, United Kingdom, Canada, and Australia both the regulatory and HTA bodies have their own independent frameworks for patient involvement while in other countries only one of the 2 stakeholders published guidance (Figure 3).
Figure 3: Overview of Patient Involvement by Regulators and HTA Bodies Within Countries
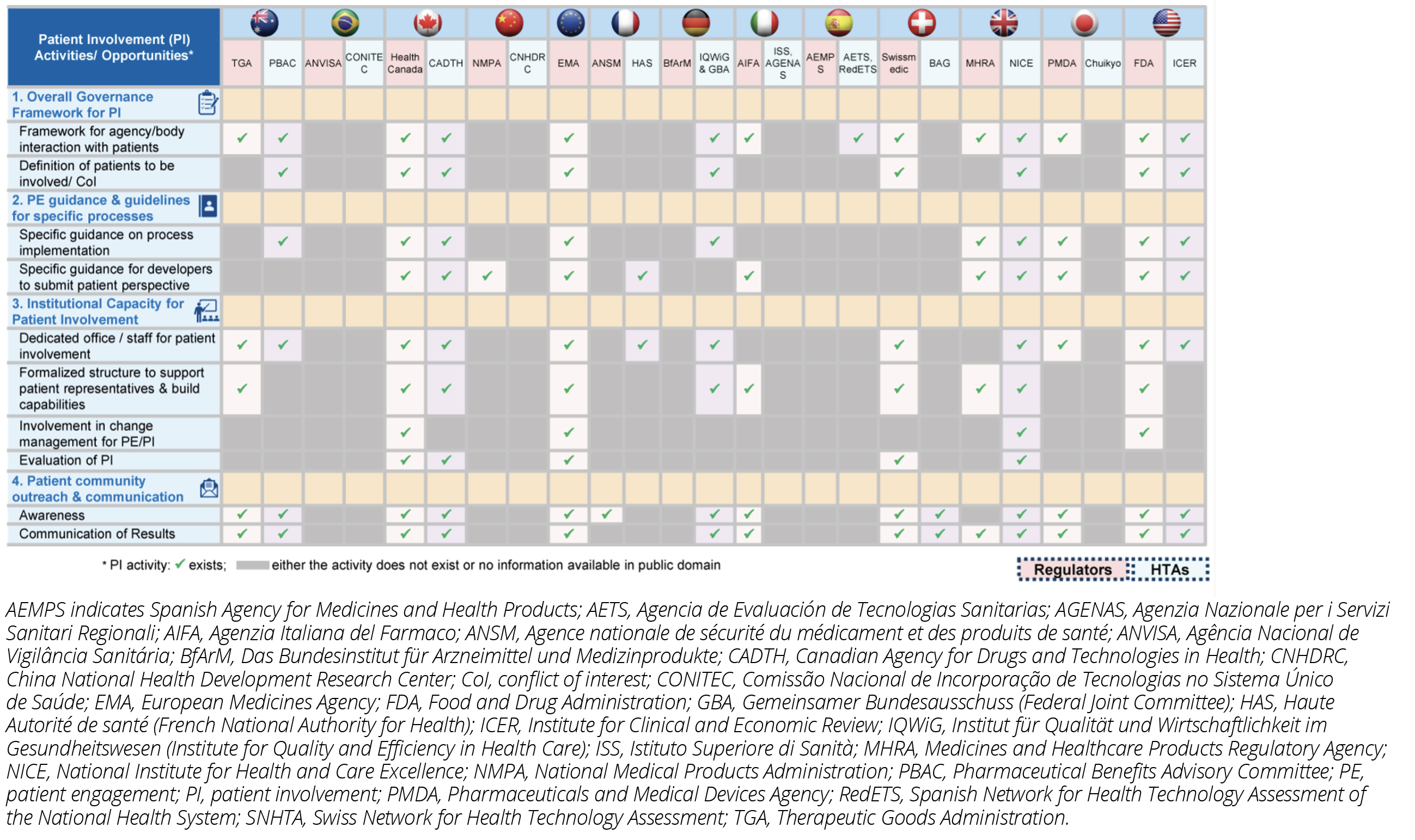
Despite publication of many guidances, no aligned methodology across borders exists that directs how patient evidence can be collected, considered, or used during decision making. There is a clear need for alignment and harmonization on patient involvement and patient evidence approaches. Greater transparency and consistency across countries is needed for an impactful implementation of a patient-focused approach to medicines development. In the absence of a consistent approach across both regulatory and HTA bodies at the national, regional, or international level, it becomes challenging to systematically improve patient involvement practices, avoid duplication of patients’ efforts, and develop broad evidence on patient experience.
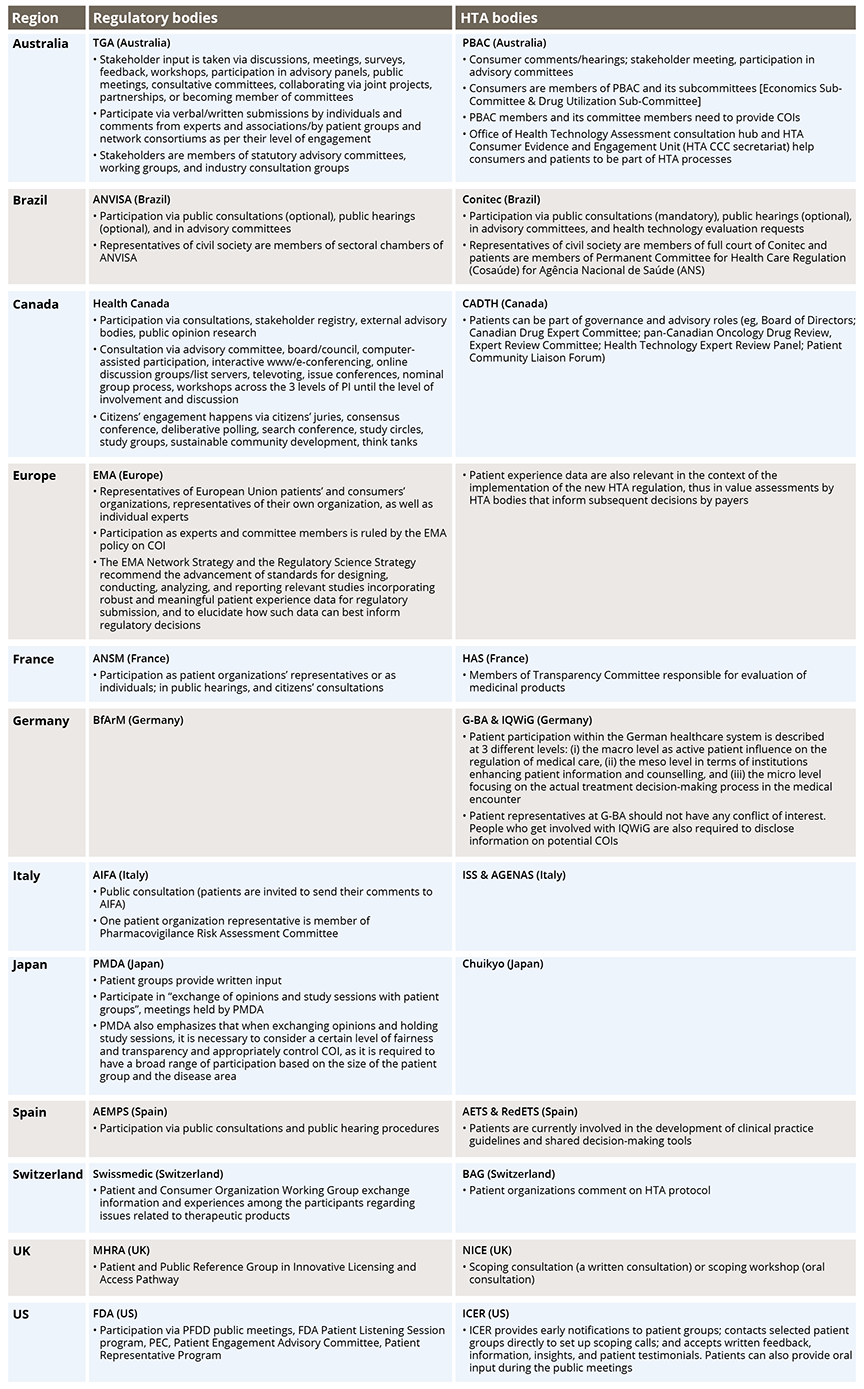
Participation in regulatory evaluations and HTA provides patients with more understanding on how new treatments are being assessed, which benefits transparency. Patients’ involvement in advisory or decision-making groups enables them to link their experiences and offer advice. In a few organizations (eg, Australia), patients are involved within stakeholder or consumer frameworks exhibiting the concept of equity to contribute in a meaningful way (Table 1). However, the variability of patient involvement for decision making can result in a potential mismatch of regulatory and HTA outcomes and introduces uncertainty into drug development decisions.
Efforts are ongoing to drive convergence across all stakeholders, fostering international collaboration (FDA and EMA established Patient Engagement Cluster workgroup in 2016) to exchange information and strengthen international collaboration on patient engagement. Health Canada became an official member of the Patient Engagement Cluster in 2022. Similar clusters could facilitate conversation on how to engage or involve patients, processes for selecting and preparing patients to participate, and discussion on goals to scale up future engagement. Several international platforms facilitate collaboration between the HTA bodies, such as the Health Technology Assessment International Patient and Citizen Involvement Group, HTA-Regulatory Interactions and Conditional Coverage, and the European network for HTA (EUnetHTA). Building synergies across regulatory and HTA bodies could improve clarity of the evidence needed and lead to decisions based on a joint understanding of patient needs, including the patient involvement provision of joint scientific advice.
Health equity is another topic in the area of involving patients, yet it differs in its purpose from involving patients in decision making. Regulators and HTA bodies are supporting incorporation of health equity. The FDA initiative, “Enhance EQUITY Initiative”, supports diversity in clinical trials and “Enhance EQUITY of Voices” acknowledges the diversity within the patient community. The involvement of and guidance on diverse populations in decision making is a large topic that would warrant additional and separate research work. ICER has published a white paper recommending key methods through which HTA can support health equity. Health equity is equally important and related, yet a slightly different topic from the active engagement of patients in decision making. The assurance that the involved patients are representative of a diverse population is indeed an area where further efforts are needed.
Table 1: Mode of Patients’ Involvement Across Organizations
Call to Action for the Future
Patients bring real-life experience. Patient involvement empowers patients to play an active role in healthcare systems and is becoming a mandatory step for the drug developers during evaluations. Systematic patient involvement ensures the relevance, legitimacy, and transparency of assessments and recommendations.
The separate processes of regulatory and HTA-related patient input result in a degree of uncertainty on the patient evidence that needs to be provided by developers and requires double the capacity from the patient community. Focusing on a standard and internationally acceptable patient involvement processes across regulators and HTA bodies would help to avoid duplication, strengthen the patient evidence, and certainly increase transparency on which data are needed and how they are used. Ideally, efforts of regulators and payers should be aligned within countries and across geographies to further enhance the level of evidence and the impact patients have in healthcare systems decisions. To progress, further research is needed to evaluate the impact of patient involvement in decision making. Simultaneously, a broader discussion is required on how patient involvement can support health equity efforts and how diverse and representative populations get involved, aiming at better health outcomes and equity. Further research will be needed to address how this equity in the involvement can be achieved to drive strong patient-informed decision making across all stakeholders and patient populations.
References
1. Food and Drug Administration. Learn About FDA Patient Engagement. Updated July 8, 2021. Accessed January 24, 2024. Learn About FDA Patient Engagement | FDA
2. European Medicines Agency. Patients and consumers. Accessed January 24, 2024. Patients and consumers | European Medicines Agency (europa.eu)
3. Therapeutic Goods Administration. Consultation and engagement. Updated March 21, 2023. Accessed January 24, 2024. Consultation and engagement | Australian Government Department of Health and Aged Care
4. Health Canada. Public Engagement: Health Canada. Updated November 2, 2022. Accessed January 24, 2024. Public Engagement: Health Canada - Canada.ca
5. Mario Melazzini, Agenzia Italiana Del Farmaco (AIFA). The Empowerment and the Engagement of citizens and patients in healthcare. Published January 26, 2017. Accessed January 24, 2024. The Empowerment and the Engagement of citizens and patients in healthcare patients in healthcare (aifa.gov.it)
6. Swissmedic. Collaboration with patient and consumer organisations. Updated 2019. Accessed January 24, 2024. Collaboration with patient and consumer organisations (swissmedic.ch)
7. Food and Drug Administration. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making. Updated June 2023. Accessed July 16, 2023. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical
8. Food and Drug Administration, Center for Devices Radiological Health. Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling. Issued August 24, 2023. Accessed July 16, 2023. www.fda.gov/media/92593/download
9. European Medicines Agency. Multi-stakeholder workshop: Patient experience data in medicines development and regulatory decision-making. Multi-stakeholder workshop: Patient experience data in medicines development and regulatory decision-making | European Medicines Agency (europa.eu). Published September 21, 2022. Accessed July 16, 2023.
10. Medicines and Healthcare products Regulatory Agency. Patient Involvement Strategy: One Year On. Published March 9, 2023. Accessed January 24, 2024. Patient Involvement Strategy: One Year On - GOV.UK (www.gov.uk)
11. Pharmaceuticals and Medical Devices Agency. Patient Centricity WG. Accessed January 24, 2024. Patient Centricity WG | Pharmaceuticals and Medical Devices Agency (pmda.go.jp)
12. Pharmaceuticals and Medical Devices Agency. Guidance on Patient Participation. Published September 7, 2022. Accessed July 16, 2023. www.pmda.go.jp/files/000243407.pdf
13. Lopes ACF, Novaes HMD, Soarez PC. Patient and public involvement in health technology decision-making processes in Brazil. SciELO Brazil - Revista de Saude Publica. 2020; 54. Published 2020. Accessed July 16, 2023. https://www.scielo.br/j/rsp/a/ypvNJdHgtgdnJqZNn5smV4K/?lang=en
14. Yan D. New China Trial Design Rules Stress Patient Needs And Experience. PINK SHEET; August 2, 2023. Accessed August 4, 2023. https://pink.pharmaintelligence.informa.com/PS148622/New-China-Trial-Design-Rules-Stress-Patient-Needs-And-Experience
15. Pharmaceutical Benefits Advisory Committee. Consumer input. Accessed January 24, 2024. Pharmaceutical Benefits Scheme (PBS) | 6.7 Consumer input
16. Canadian Agency for Drugs and Technologies in Health. Patient and Community Engagement. Updated January 11, 2019. Accessed January 24, 2024. Patient and Community Engagement | CADTH
17. National Institute for Health and Care Excellence. Patient and public involvement policy. Accessed January 24, 2024. Patient and public involvement policy | Public Involvement Programme | Public involvement - putting you at the heart of our work | NICE and the public | NICE Communities | About | NICE
18. European Cancer Patient Coalition (ECPC). HTA & Patient Involvement – Examples from EU Member States. Published 2020. Accessed January 24, 2024. HTA & Patient Involvement (ecpc.org)
19. Haute Autorité de santé. Transparency Committee. Accessed January 24, 2024. Haute Autorité de Santé - Transparency Committee (has-sante.fr)
20. Toledo-Chávarri A, Pego YT, Rodrigo ER, Roteta NI, Novella-Arribas B, Edo MJV, Benavent PG, Puigdomenech E, Linde JMM. Evaluation of patient involvement strategies in health technology assessment in Spain: the viewpoint of HTA researchers. Cambridge University Press. Published September 11, 2020. Accessed January 24, 2024. Evaluation of patient involvement strategies in health technology assessment in Spain: the viewpoint of HTA researchers | International Journal of Technology Assessment in Health Care | Cambridge Core
21. Gemeinsamer Bundesausschuss. Patient representative spokespersons. Updated 2021. Accessed January 24, 2024. Patient representative spokespersons (g-ba.de)
22. Institute for Clinical and Economic Review. History & Impact. Accessed January 24, 2024. Our History & Impact | Who We Are | ICER
23. Global Alliance for Patient Access. Patient Engagement in Health Technology Assessment - Practices and Principles for Europe and the United States. Accessed July 16, 2023. https://gafpa.org/wp-content/uploads/2021/05/GAfPA_Patient-Engagement-in-Health-Technology-Assessment_Dec-2020.pdf
24. Facey KM, Hansen HP, Single ANV. Patient Involvement in Health Technology Assessment. Singapore: Adis Singapore; 2017. Accessed July 16, 2023. https://link.springer.com/book/10.1007/978-981-10-4068-9

