A Review of 2021’s Top Global HEOR Trends: Perspectives From the ISPOR Global Groups
Robert Selby, MBA, Director, Global Networks, ISPOR, Lawrenceville, NJ, USA
To better understand how global health economics and outcomes research (HEOR) priorities are shifting through the COVID-19 pandemic, ISPOR conducted a survey among leaders within some of the ISPOR global groups to assess their perspectives on what the top HEOR trends are for the regions in 2021. As part of their annual meeting agendas, executive committee members of the ISPOR Asia Consortium, Latin America Consortium, Central and Eastern Consortium, Arabic Networks, and Africa Networks were asked to share the top 3 HEOR topics for their respective regions or work areas. The survey was not intended to be an exhaustive investigation but rather an interesting exercise to shed more light on global perspectives from ISPOR global leaders.
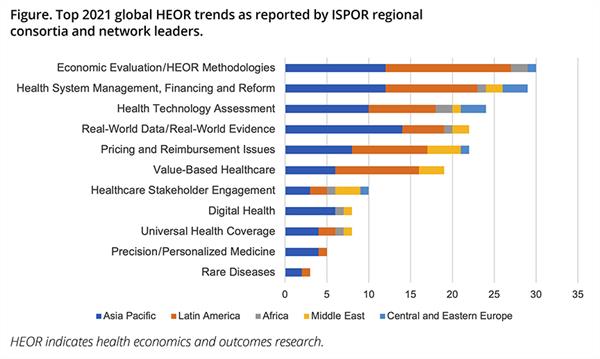
The Figure below shows the distribution of top trending topics by region as reported by the leaders.
Figure. Top 2021 global HEOR trends as reported by ISPOR regional consortia and network leaders.
Methodology
A total of 101 people were surveyed with 67 responding. The Asia Pacific region accounted for nearly 45% of the total responses, with 34% from Latin America, 9% from Africa, 9% from the Central and Eastern Europe region, and 10% from the Middle East. The survey yielded 160 individual topic suggestions, with 50% of the suggestions from the Asia Pacific region, 35% from Latin America, 9% from the Middle East, 6% from the Central and Eastern Europe region, and 6% from Africa. (Note: It is acknowledged that this exercise is limited by the uneven distribution of responses from the regions, which presents bias in the results toward the response-heavy regions, namely Asia Pacific and Latin America.)
The individual topic responses were grouped into 11 categories. Recommendations relating to general HEOR methodologies and particularly, economic evaluation practices were grouped into the category “Economic Evaluation/HEOR Methodologies.” There were also broader categories encompassing health system management, financing and reform, and pricing and reimbursement issues. “Value-Based Healthcare” was listed as a separate topic from “Pricing and Reimbursement Issues,” due to its emergence as a widely acknowledged novel concept that also covers healthcare delivery.
The Table shows examples of specific topic suggestions within the broader categories.
Table. Top 2021 HEOR global trends and subtopics as reported by ISPOR regional consortia and network leaders.
 Adapting HEOR Core Methodologies to New Paradigm
Adapting HEOR Core Methodologies to New Paradigm
As healthcare and health systems undergo radical transformation through the effects of the COVID-19 pandemic and disruptive technological innovation, ISPOR regional leaders have prioritized the adaptation of economic evaluation and other core HEOR methodologies to be more fit-for-purpose for the real world. Crucially, this topic has also been identified as a strategic priority for the recently published ISPOR Science Strategy. Specific issues raised include revisiting traditional measures that estimate quality of life and health state utility, specifically for children and other special populations, and ICER thresholds and assumptions surrounding opportunity cost versus willingness to pay (societal vs individual). More generally, countries in need of foundational HEOR capacity require the development of national pharmacoeconomic guidelines, and countries with existing guidelines should update them regularly. Recently, Hungary’s National Department of Health established a Guideline Revision Committee to update the “Guideline for Economic Evaluations in Healthcare in Hungary,” and to lay out new recommendations for a revised cost-effectiveness threshold in the country.1 In China, the most recent iteration of pharmacoeconomic guidelines was published in 2020, which updated and added to foundational frameworks in place from the previous version, drawing on the latest research in pharmacoeconomic evaluation, adapting practices to a societal view, and incorporating a broader range of therapies including traditional Chinese medicines. However, developing accurate health utility measures for Chinese subpopulations has remained a challenge for research going beyond traditional cost-effectiveness analysis.2
Strengthening Regional Health Systems
Even prior to the pandemic, many countries’ health systems were struggling to deliver optimal health outcomes for patients in the face of severe budget constraints, given the growing burden of chronic disease and the proliferation of expensive innovative therapies. The pandemic has exacerbated these challenges, simultaneously derailing economic growth and putting tremendous financial pressure on public health entities to finance crisis response and vaccination programs. Many countries have had to rebuild their health systems, make them more efficient, and find new ways to prioritize spending. In Latin America, a need for novel financing mechanisms for health systems is leading to innovative approaches, including results-based financing initiatives such as Argentina’s Plan Nacer/Programa Sumar, and impact funds supported by nonprofit entities, corporations, and investment banks such as the UNICEF Bridge Loan Fund.3 Centralized procurement has emerged as a key tool for cost savings, with China implementing their “4+7 Plan” for generics and Mexico developing a national compendium that established standard formulary bidding, procurement and health technology assessment (HTA) processes across the country’s health institutions.4,5 In the Middle East, public-private partnerships are increasingly seen as a viable strategy to improve the performance of health systems by bringing together the best characteristics of the public and private sectors to improve efficiency, quality, and innovation.6
Pricing and Reimbursement Issues
Pricing and reimbursement remain a highly important topic for global leaders, particularly around the aspects of transparency and access to therapies. In particular, how governments are enabling broader access to COVID-19 therapies and how the pricing is determined are of great interest. In a bid to support broader access to COVID-19 vaccines in lower- and middle-income countries, governments in India and South Africa raised a proposal last year for consideration by the World Trade Organization to waive intellectual property protections on COVID-19 vaccines and treatments. No formal decision has been made yet on this by the council, with further discussion expected at their meeting in June. As more countries reassess their positions on this issue, the ongoing discussions could provide impetus for reorientation toward greater global collaboration on equitable access to therapies.7
Other countries are taking proactive steps to update their own national reimbursement drug lists. In China, the National Healthcare Security Administration in 2020 established a dynamic update mechanism of the National Reimbursement Drug List, which provides for annual reviews and updates. A national drug price negotiation mechanism between the government and the pharmaceutical companies was also formally introduced in China in 2017, which has centralized negotiations around pricing and reimbursement.8 The introduction of high-cost innovative therapies, including curative and gene therapies, is also necessitating novel approaches to reimbursement and access, including managed-entry/risk-sharing schemes, mortgage or subscription payment models, indication-specific or value-based pricing, and other value-based contracting approaches.9 In South Korea, financial-based risk-sharing agreements have increased markedly in prevalence (there were 11 risk-sharing agreements in 2016 compared to 35 risk-sharing agreements in 2020), and a coverage expansion policy by the National Health Insurance program has led to an increase in the overall coverage rates for interventions, where provisions are made for selective or preliminary coverage of novel therapies with subsequent evidentiary development over a 3- to 5-year timeline.10
HTA’s New Challenges
HTA has faced new challenges in the wake of the pandemic, forcing traditional processes and roles to adapt to new settings and requirements. HTA bodies have had to rapidly evaluate and approve interventions that may not have sufficient clinical or cost-effectiveness data available. In Latin America, a lack of readily available local evidence has led to changes in evidence sourcing, with the recently established Regional Database of Health Technology Assessment Reports of the Americas (developed jointly between Health Technology Assessment Network of the Americas and the Pan American Health Organization) serving as an important resource for the region. In Mexico, HTA is being used as a tool to support prioritization and rational use of health resources and to maintain sustainable healthcare budgets.
Specific emphasis is being placed on expanding hospital-based HTA to cover all medical technologies and treatment pathways in the hospital setting.5 Many countries are in the process of establishing new HTA units or agencies or strengthening existing HTA bodies. Japan’s new HTA unit, the Center for Outcomes Research and Economic Evaluation for Health, which was established in April 2019, has begun releasing their first public economic evaluation reports after an extensive pilot program.11 In the Ukraine, efforts are ongoing to strengthen processes and legal frameworks to make HTA more sustainable, reliable, and independent, and clarify the role of HTA in the transition towards a national positive list for reimbursement.12 Other issues, such as the transferability of HTA across jurisdictions and HTA of medical devices, remain important priorities for global HEOR.
Additional Topic Considerations
Subsequent topics seemed to vary in importance across the different regions. In the Asia Pacific, real-world data, digital health, and precision medicine were ranked higher in priority, while in Latin America, value-based healthcare and universal health coverage ranked higher. In Europe, the Middle East, and Africa, healthcare stakeholder engagement was ranked higher, specifically referring to patient advocacy groups, engagement, and multistakeholder involvement in healthcare decision making. These variances could reflect the level of capacity or development in the regional systems or could just be a product of the survey population’s skew. Further investigation along these lines could yield interesting results.
We welcome your further thoughts and input! To send your comments about this article or for more information and to join ISPOR global groups, please contact globalgroups@ispor.org. •
Acknowledgment:
Special thanks to members of the ISPOR Asia Consortium, Latin America Consortium, Central and Eastern Consortium, and Arabic Network and Africa Networks for contributing their recommendations and comments to this survey.
References
1. HEOR Activities in Hungary in 2020–ISPOR Hungary Chapter Update. ISPOR News Across Europe, Middle East, and Africa. ISPOR.org. Published February 2021. https://press.ispor.org/emea/index.php/2021/02/12/heor-activities-in-hungary-in-2020-ispor-hungary-chapter-update/. Accessed April 16, 2021.
2. Yue X, Li Y, Wu J, Guo JJ. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China, Value Health Reg Issues. 2021.24(C):1-5. https://doi.org/10.1016/j.vhri.2020.07.580.
3. Guarin D, Lunes R, Uribe M. Generando Inversiones para la Atención en Salud en América Latina. ISPOR.org. Published April 12, 2021. https://www.ispor.org/conferences-education/education-training/webinars/webinar/generando-inversiones-para-la-atenci%C3%B3n-en-salud-en-am%C3%A9rica-latina. Accessed April 16, 2021.
4. Yue X. “4+7” Drug Procurement Reform in China. China National Health Development Research Center, Beijing, China. Published 2019. https://www.cgdev.org/sites/default/files/CGD-procurement-background-china-case.pdf. Accessed April 16, 2021.
5. HTA’s Evolving Role Through the COVID Pandemic and Beyond–Virtual ISPOR 52nd HTA Roundtable–Latin America. ISPOR News Across Latin America. ISPOR.org. Published February 2021.https://press.ispor.org/LatinAmerica/2021/02/htas-evolving-role-through-the-covid-pandemic-and-beyond-virtual-ispor-52nd-hta-roundtable-latin-america/. Accessed April 16, 2021.
6. ISPOR Arabic Network Forum Report: Effect of Public and Private Partnership (PPP) in Healthcare Financing to Improve Healthcare Outcomes. ISPOR News Across Europe, Middle East, and Africa. ISPOR.org. Published February 2021. https://press.ispor.org/emea/index.php/2021/02/12/ispor-arabic-network-forum-report-effect-of-public-and-private-partnership-ppp-in-healthcare-financing-to-improve-healthcare-outcomes/. Accessed April 16, 2021.
7. Green A.TRIPS Waiver Tripped Up in WTO by “Third Way.” Devex. Published March 5, 2021. https://www.devex.com/news/trips-waiver-tripped-up-in-wto-by-third-way-99329. Accessed April 16, 2021.
8. Dynamic Updates of National Reimbursement Drug List (NRDL) in China 2020. ISPOR News Across Asia. ISPOR.org. Published February 2021. https://press.ispor.org/asia/index.php/2021/02/05/dynamic-updates-of-national-reimbursement-drug-list-nrdl-in-china-2020/. Accessed April 16, 2021.
9. Comer B. Six Drug Pricing Models Have Emerged to Improve Product Access and Affordability. pwc. Published September 23, 2019. https://www.pwc.com/us/en/industries/health-industries/library/6-drug-pricing-models.html. Accessed April 16, 2021.
10. Kim SY, Pilasant S. New Developments in Health Technology Assessment in Asia Pacific. ISPOR.org. Published April 2021. https://www.ispor.org/conferences-education/education-training/webinars/webinar/new-developments-in-health-technology-assessment-in-asia-pacific. Accessed April 16, 2021.
11. The First Reports of the Cost-Effectiveness Evaluation Were Released. C2H. Published March 31, 2021. https://c2h.niph.go.jp/en/info/news/20210331/index. Accessed April 16, 2021.
12. Health Technology Assessment Experts Hold Policy Dialogue at National Forum in Ukraine. MSH. Published October 5, 2021. https://www.msh.org/news-events/stories/health-technology-assessment-experts-hold-policy-dialogue-at-national-forum-in. Accessed April 16, 2021.
Explore Related HEOR by Topic

