Benefit-Sharing Programs: What Role May They Play in Supporting Cost-Effective Prescribing Practices for High-Cost Biologics?
Teresa Barcina Lacosta, MSc, PharmD, KU Leuven, Leuven, Belgium; Arnold G. Vulto, PharmD, PhD, Erasmus University Medical Center, Rotterdam, The Netherlands; Adina Turcu-Stiolica, PhD, University of Medicine and Pharmacy of Craiova, Craiova, Romania; Isabelle Huys, PhD, KU Leuven, Leuven, Belgium; Steven Simoens, MSc, PhD, KU Leuven, Leuven, Belgium
Making medicines more accessible: the role of generic and biosimilar medicines
Recent analyses on the evolution of the global medicines market show a growing trend in medicine use and spending across all disease areas. Especially in oncology and in the field of immune-mediated inflammatory diseases, the increased use of high-cost biologics has become an important driver of pharmaceutical spending.1 According to the Organization for Economic Co-operation and Development (OECD), multiple countries have raised concerns about their ability to reconcile access with spending efficiency and sustainability.2
"According to the OECD, multiple countries have raised concerns about their ability to reconcile access with spending efficiency and sustainability."
The urgency to address these concerns is even higher in low- and middle-income countries, where significant access delays occur throughout the care continuum.3 In this context, the market entry of more affordable non-innovator generic and biosimilar medicines represents an opportunity to induce price competition, increase spending efficiency, and expand access to medications while maintaining the quality-of-care standards. The World Health Organization (WHO) pre-qualification and listing of generics and biosimilars has supported the use of these medicines worldwide.4 According to WHO data, the introduction of antiretroviral generic medicines allowed scaled-up access to these therapies globally.5 Likewise, the recent WHO listing of biosimilars for essential medicines such as trastuzumab is expected to increase global patients’ access to cancer care.4,6
Despite the role that generic and biosimilar medicines play in supporting patients’ access to treatments, multiple factors determine prescribing choices and the selection of “best-value” pharmaceuticals (generally generics and biosimilars) is not always prioritized. In this commentary, we examine the role that benefit-sharing strategies may play in supporting cost-effective prescribing practices for biologics across Europe.
Benefit-sharing initiatives can promote the cost-effective prescribing of biologics
Barriers have existed and still exist to the acceptance and use of biosimilars. Prescribers and patients have historically raised concerns about the safety and efficacy of these medicines, especially around the safety of switching and substituting biosimilars with the originator product or with other biosimilars. Although uncertainties surrounding these aspects have been addressed after more than 10 years of biosimilars market availability in Europe,7,8 prescribers and patients still highly value the possibility to choose between originator and biosimilar products.9
To control spending in pharmaceuticals, diverse policies have been implemented to support the use of best-value biologics (prescription quotas, benefit-sharing initiatives, etc), and to disincentivize prescribing less affordable biologics (reimbursement restrictions and budget caps). Experience tells us that policies limiting the reimbursement of pharmaceuticals, as well as policies applying prescription quotas, are perceived negatively by certain stakeholders. Across Europe, these policies have been generally applied in combination with (1) educational campaigns on biosimilars, and (2) benefit-sharing (gainsharing) initiatives aimed at generating consensus on the importance of promoting cost-effective prescribing practices for biologics. The combination of these strategies can increase the willingness of healthcare professionals (HCPs) and patients to use biosimilars, especially if it is communicated that this would lead to higher access to treatments, reduced healthcare costs, and general societal benefits.9
Although there are multiple examples in the literature of educational initiatives for biosimilars, little is known about where and how to implement benefit-sharing programs. Benefit-sharing programs can be defined as incentive programs that promote the use of best-value biologics by motivating changes in prescribing practices. The aim of these programs is to generate savings that can be shared among the stakeholders involved (eg, health authorities/payers/insurers, hospital financial departments, healthcare professionals) and that can be reinvested to fund innovation, increase patients’ access to treatments, and make quality of care improvements.
Our recent study in BioDrugs provides a detailed inventory of benefit-sharing cases.10 Based on these cases, it has been possible to (1) identify challenges that institutions face when implementing benefit-sharing programs and (2) formulate best-practice recommendations for benefit sharing. With the current commentary, we aim to increase policy makers’ awareness of these aspects. Our main goal is to support informed decision making for institutions that may implement benefit-sharing programs in the future.
The promise of implementing benefit-sharing programs: implications for key stakeholders
To date, multiple countries across Europe have launched benefit-sharing initiatives for biologics (ie, the United Kingdom, France, Germany, Ireland, Italy, The Netherlands, Portugal, and Sweden). These examples showed us a set of characteristics that are generally pursued by organizers of benefit-sharing programs. Most benefit-sharing programs implemented in Europe have aimed to:
• Set prescription objectives for best-value biologics
• Evaluate prescribers’ compliance with the set prescription objectives
• Generate savings and reinvest them according to the needs of the stakeholders who produced them
• Establish pathways for savings reinvestment that enable funding health services and quality of care improvements
This general scope of benefit-sharing initiatives can be illustrated, for example, by the Irish National benefit-sharing program.11 In Ireland, the benefit-sharing initiative was focused on the TNF-α inhibitor products adalimumab and etanercept. The first step in the implementation of this initiative was to establish criteria for the identification of best-value biologics. The application of these criteria led to the selection of various adalimumab and etanercept biosimilars as best-value products. For these products, an 80% prescription objective was set to be achieved in a defined timeframe. The hospital clinical departments that initiated or switched eligible patients to best-value biologics received €500 of the resulting savings per patient. One year after the implementation of the Irish benefit-sharing initiative, the uptake of best-value products had considerably increased and savings amounted to €22.7M. Approximately 16% of the generated savings were returned to the hospital clinical departments for reinvestment into improvements in the provision of care (eg, increased infusion room’s capacity for intravenous formulations, development of electronic patient registries).
"Despite the role that generic and biosimilar medicines play in supporting patients’ access to treatments, multiple factors determine prescribing choices and the selection of best-value pharmaceuticals (generally generics and biosimilars) is not always prioritized."
The Irish benefit-sharing initiative represents just one example of the benefits to be achieved via benefit sharing. In Figure 1 we provide a more comprehensive list of potential benefits that can be realized for the different stakeholder groups involved. However, the examination of benefit-sharing cases across Europe suggests that the promise of implementing benefit-sharing programs (Figure 1) has only been partially delivered. In some cases, patients were not informed about the outcomes achieved via benefit sharing and about how their participation in these programs improved their care. Furthermore, HCPs and clinical departments were not always able to participate in decisions regarding the reinvestment of savings. This has been partly due to time constraints that hindered the preparation of formal reinvestment plans and to the urgency to reallocate savings to cover expenses in other care areas.
Figure 1. Outline of benefits that can potentially be realized via the implementation of benefit-sharing programs for best-value biologics. For each stakeholder group, we provide a list of the most relevant benefits to be achieved.
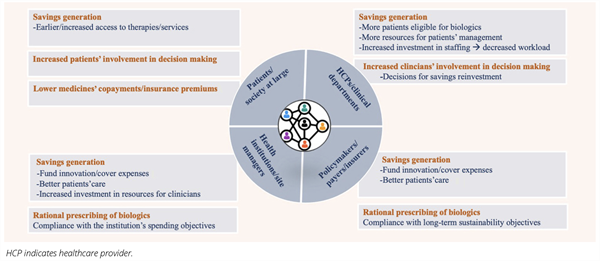
These aspects evidence a general lack of transparency regarding financial flows. Also, the evaluation of the success of implemented benefit-sharing programs was opaque in some instances. Therefore, it has been uncertain how to determine the direct impact of benefit-sharing programs on patients’ care. We include in Table 1 a summary of challenges faced by institutions when implementing benefit-sharing programs. The relative relevance of these challenges for each implementation setting has primarily depended on factors like the degree of centralization of the healthcare system; the available communication channels between managers, HCPs, and patients; the policy environment; and the specific implementation timeframe.
Table 1. Overview of most relevant challenges faced by institutions when implementing benefit-sharing programs for biologics.
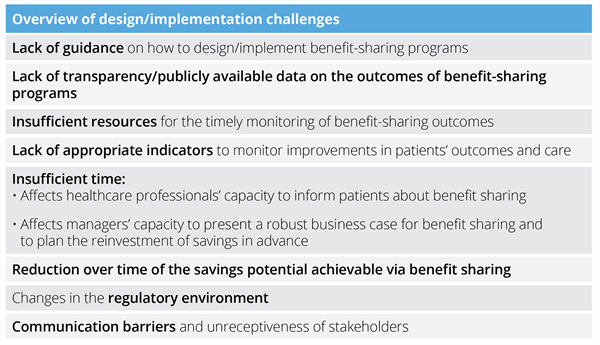
The challenges associated with the implementation of benefit-sharing programs have raised questions about how to actively engage all the stakeholder groups in decision making, and how to fully deliver on the promise of benefit sharing in the future.
Strategies moving forward to optimize the implementation of benefit-sharing initiatives
Our research conducted on benefit-sharing cases in Europe shows that there is no one-size-fits-all best approach for the successful design and implementation of benefit-sharing programs. As discussed before, the conditions for benefit sharing need to adapt to the specific socioeconomic background of the country, the characteristics of the healthcare system, the clinical context, and the regulatory and political environment. A point of practical consideration is that if the use of best-value biologics is already optimal or if the savings potential associated with the use of best-value pharmaceuticals is low, it may not be economically advantageous for the payer to apply benefit-sharing strategies. Also, in countries where the spending on biologics is low, the application of benefit-sharing initiatives may not be relevant. These aspects are to be considered carefully by future benefit-sharing implementers.
"The challenges associated with the implementation of benefit-sharing programs have raised questions about how to actively engage all the stakeholder groups in decision making."
While acknowledging the importance of considering the particularities of each implementation setting, it is possible to formulate some general best practice recommendations. To realize the full potential of benefit-sharing programs, greater attention should be paid to the following 5 aspects: (1) informing HCPs and institutions of the principles of benefit sharing in advance; (2) setting up and monitoring success indicators for benefit-sharing programs on a timely basis; (3) including quality of care parameters as success indicators; (4) establishing clear pathways for the transparent distribution and reinvestment of savings; and (5) transparently communicating with patients about the outcomes of benefit-sharing programs.
These key recommendations, extracted from the observation of benefit-sharing cases in Europe, could be applicable to other jurisdictions. However, institutions implementing benefit-sharing initiatives in the future should determine first whether the socioeconomic/ regulatory environment of their countries and the healthcare system organization would make this implementation feasible and advantageous. We hope that the learnings outlined in this commentary can serve as a starting point to guide future implementers of benefit-sharing programs.
References
1. IQVIA Institute for Human Data Science. Global Medicines Spending and Usage Trends, Outlook to 2025. 2021. Accessed June 16, 2022. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-medicine-spending-and-usage-trends-outlook-for-2025/iqvia-institute-global-medicines-and-usage-trends-to-2025-0421-forweb.pdf
2. OECD. Better Policies for Better Lives. Addressing Challenges in Access to Oncology Medicines. Analytical Report. 2020. Accessed June 17, 2022. https://www.oecd.org/health/health-systems/Addressing-Challenges-in-Access-to-Oncology-Medicines-Analytical-Report.pdf
3. Brand NR, Qu LG, Chao A, Ilbawi AM. Delays and barriers to cancer care in low- and middle-income countries: a systematic review. Oncologist. 2019;24(12):e1371-e80. doi:10.1634/theoncologist.2019-0057
4. World Health Organization (WHO). World Health Organization Model List of Essential Medicines. 22nd List. 2021. Accessed June 17, 2022. https://apps.who.int/iris/bitstream/handle/10665/345533/WHO-MHP-HPS-EML-2021.02-eng.pdf
5. World Health Organization (WHO). Generic medicines. Interchangeability of WHO-prequalified generics. WHO Drug Information. 2016;30(3). Accessed July 18, 2022. https://apps.who.int/iris/handle/10665/331014
6. McBride A, Balu S, Campbell K, Bikkina M, MacDonald K, Abraham I. Expanded access to cancer treatments from conversion to neutropenia prophylaxis with biosimilar filgrastim-sndz. Future Oncol. 2017;13(25):2285-95. doi:10.2217/fon-2017-0374
7. Barbier L, Vulto AG. Interchangeability of biosimilars: overcoming the final hurdles. Drugs. 2021;81(16):1897-903. doi:10.1007/s40265-021-01629-4
8. Kurki P, Barry S, Bourges I, Tsantili P, Wolff-Holz E. Safety, immunogenicity and interchangeability of biosimilar monoclonal antibodies and fusion proteins: a regulatory perspective. Drugs. 2021;81(16):1881-96. doi:10.1007/s40265-021-01601-2
9. Barbier L, Simoens S, Vulto AG, Huys I. European stakeholder learnings regarding biosimilars: part i-improving biosimilar understanding and adoption. BioDrugs. 2020;34(6):783-96. doi:10.1007/s40259-020-00452-9
10. Barcina Lacosta T, Vulto AG, Turcu-Stiolica A, Huys I, Simoens S. Qualitative analysis of the design and implementation of benefit-sharing programs for biologics across Europe. BioDrugs. 2022. doi:10.1007/s40259-022-00523-z
11. Duggan B, Smith A, Barry M. Uptake of biosimilars for TNF-α inhibitors adalimumab and etanercept following the best-value biological medicine initiative in Ireland. Int J Clin Pharm. 2021. doi:10.1007/s11096-021-01243-0

