Sticking With It: Assessing Adherence Rates in Real-World Studies Using Digital Technologies
Akosua Ofori, MPH, Oktawia Borecka, PhD, Sam Llewellyn, MPH, Vitaccess, London, England, UK
Introduction
Longitudinal observational studies play a central role in advancing understanding of the onset and progression of physical and mental health conditions in the real world1 and help with extrapolating data obtained in randomized controlled trials.2 Once recruited, participants in longitudinal studies are asked to adhere to data capture activities at specified timepoints. Leaders of pharmaceutical and biotech organizations have identified patient recruitment—including participant retention—as the main challenge in real-world evidence generation.3 Ensuring participant adherence or engagement in these studies is important to avoid attrition (ie, participants leaving the study or being lost to follow-up) and poor completion (ie, remaining in the study but not providing complete data). Poor adherence can reduce the generalizability of outcomes and the statistical power to detect effects of interest.
As the use of digital technologies increases, researchers are able to gather data from patients using online surveys and questionnaires accessible through mobile phones and computers. However, some data show that such digital studies can be affected by poor participant adherence.4 Adherence is particularly important for data capture activities using digital technologies that are completed without the supervision of clinical staff at physical study sites.
This article assesses participant adherence rates in real-world studies, with a focus on those using digital technologies.
Adherence in real-world studies using digital technologies
The adherence rates to data capture activities that are reported in the literature vary considerably. A targeted review of publications involving real-world studies using digital technologies from the last 10 years on PubMed and Google Scholar yielded 14 relevant results.5
Among the 9 studies with daily data collection, adherence to data capture activities ranged from 9% to 96%. Two studies implemented weekly data collection, with adherence ranging from 63% to 84%. Among the 3 studies with the least frequent data collection, adherence by the end of the study ranged from 1% to 38%. The total data collection period of all studies varied; the studies with the most frequent data collection (daily) had the shortest total data collection period, ranging from 7 to 141 days. However, the studies with the least frequent data collection (ranging from once per 3 months to yearly) had the longest total data collection period, ranging from 1 to 4.5 years. A full breakdown of the types of study participants, the locations, and key findings can be found in Table 1.5
Table 1: Overview of adherence rates in real-world studies using digital technologies5
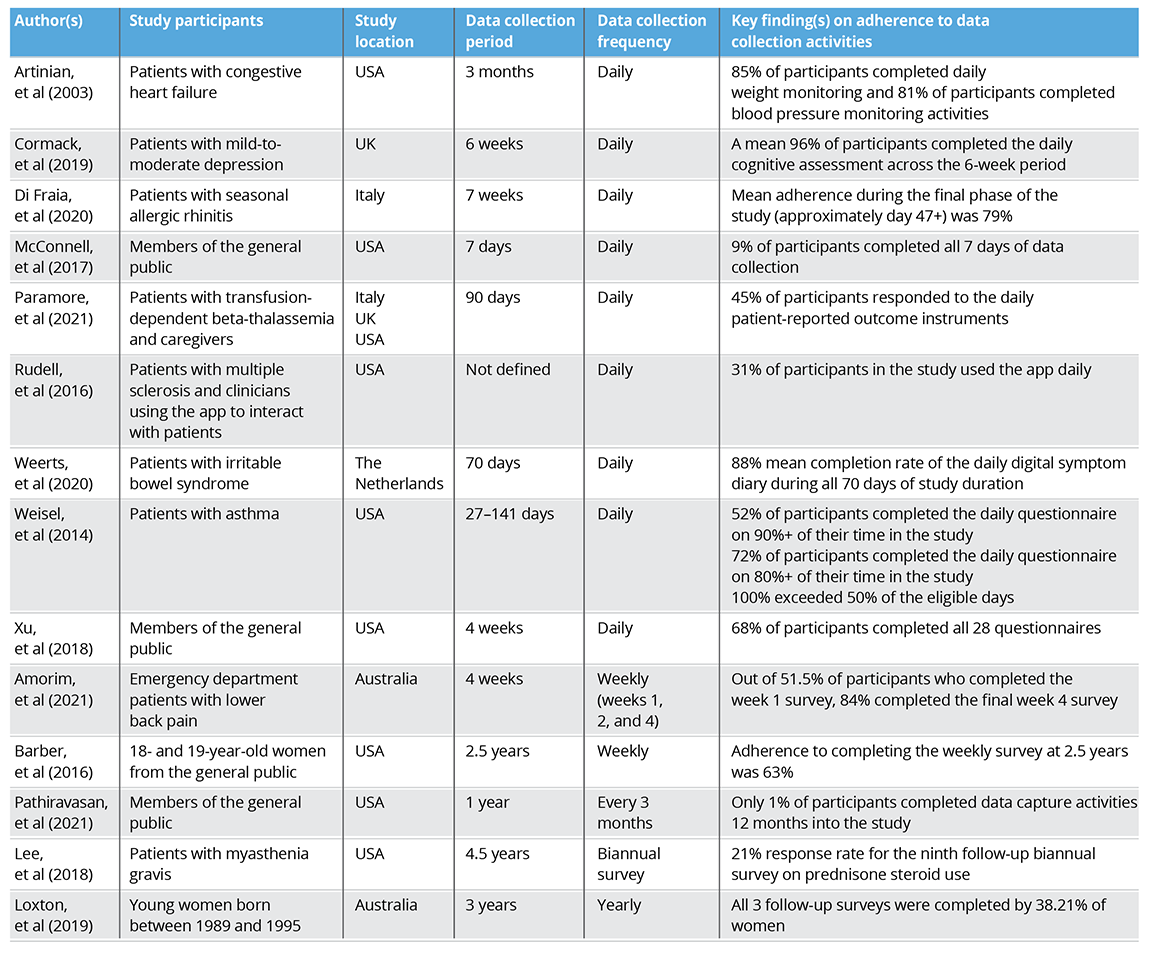
Overall, no trend was observed to indicate that adherence was dependent on specific factors, including data collection frequency, study duration, or location. The number of studies reviewed in this article might not have been large enough to draw robust conclusions. A systematic review or meta-analysis may help to further explore whether specific study characteristics impact adherence rates.
"Leaders of pharmaceutical and biotech organizations have identified patient recruitment—including participant retention—as the main challenge in real-world evidence generation."
Potential strategies to improve adherence
Including participants in the development process may increase the likelihood that they will have greater engagement once the study launches. Participants can be involved in the development of real-world studies by providing input in study design or reviewing participant-facing material,7-9 contributing to UI/UX design or acceptance testing.10-15
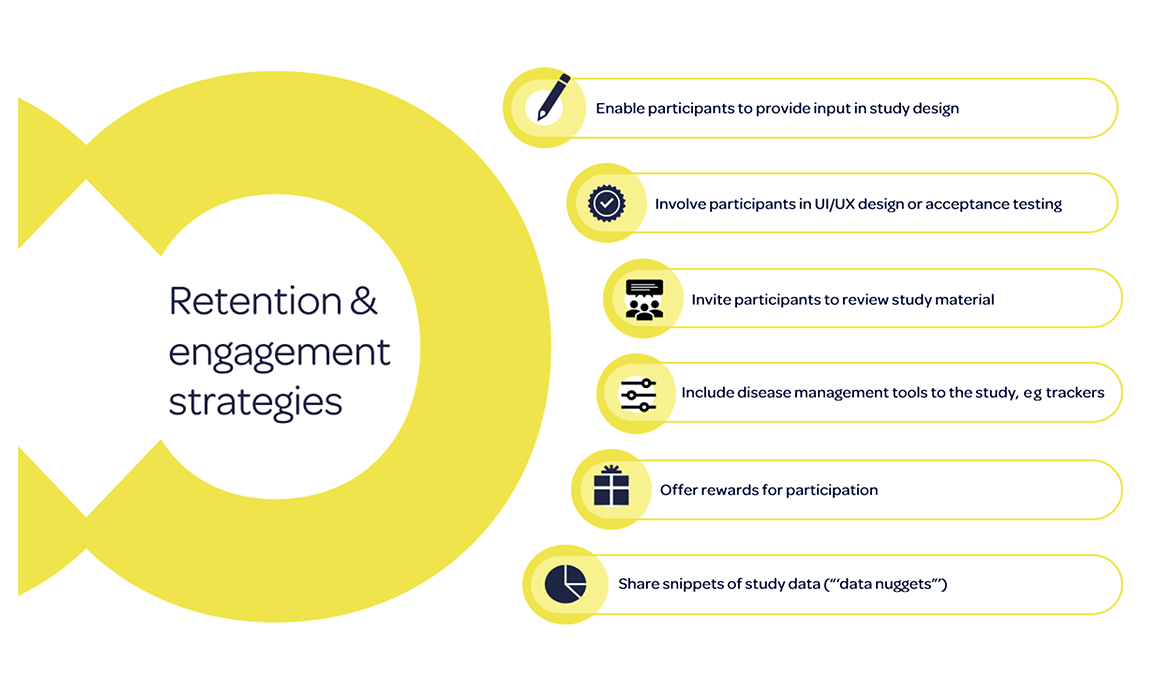
Additional strategies can be implemented during the study, particularly for real-world research using digital technologies, as summarized in the figure.6 Disease management tools such as trackers can be implemented to improve completion rates,16 and offering rewards for participation can improve engagement.17,18 Of the studies reviewed in the literature review, 6 reported offering participants incentives for completion of data capture activities.5 None, however, explored whether incentives impacted adherence to data capture activities.5 Finally, participants could also be sent regular “data nuggets” with snippets of study data to encourage continued adherence.19
Figure. Retention and engagement strategies.
Conclusion
Real-world research is crucial when creating a broad and granular picture of a disease or research area.2 In order to draw robust, generalizable conclusions from real-world studies, participant adherence to data capture activities is vital. Several methods could be utilized during the design and execution of real-world studies that can encourage adherence, such as patient cocreation and incentives.
"In order to draw robust, generalizable conclusions from real-world studies, participant adherence to data capture activities is vital."
Future research could also focus on a detailed investigation of the relationship between incentives and adherence to data capture activities, as well as other means of patient involvement in the study design. It is important to understand the factors that may affect adherence, in order to inform the design of future studies.
References
1. Taur SR. Observational designs for real-world evidence studies. Perspect Clin Res. 2022;13(1):12-16. doi:10.4103/picr.picr_217_21
2. Katkade VB, Sanders KN, Zou KH. Real-world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc. 2018;11:295-304. doi:10.2147/JMDH.S160029
3. Essex L. RWD/RWE top named Opportunity Area in PPD Pharmaceutical Industry survey. The Evidence Base. October 25, 2022. Accessed September 7, 2023. https://www.evidencebaseonline.com/rwd-rwe-top-named-opportunity-area-in-ppd-pharmaceutical-industry-survey/
4. Pratap A, Neto EC, Snyder P, et al. Indicators of retention in remote digital health studies: a cross-study evaluation of 100,000 participants. npj Digit Med. 2020;3,21. doi:10.1038/s41746-020-0224-8
5. Borecka O, Ofori A, Llewellyn S. Please complete your surveys: a targeted review of adherence rates in real-world studies using digital technologies. Presented at ISPOR Europe 2022; November 2022; Vienna, Austria.
6. Llewellyn S, Amini F. Should I stay or should I go? Patient engagement and retention in real-world studies. December 14, 2022. Accessed September 15, 2023. https://vitaccess.com/blogs/patient-engagement-in-real-world-studies/
7. Goodson N, Wicks P, Farina C. Commentary: An industry perspective on the importance of incorporating participant voice before, during, and after clinical trials. Trials. 2022;23(1). doi:10.1186/s13063-022-06905-6
8. Crocker JC, Ricci-Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. 2018.363:k4738 doi:10.1136/bmj.k4738
9. Levitan B, Getz K, Eisenstein EL, et al. Assessing the financial value of patient engagement: a quantitative approach from CTTI’s patient groups and Clinical Trials Project. Ther Innov Regul Sci. 2017;52(2):220-229. doi:10.1177/2168479017716715
10. Petracca F, Tempre R, Cucciniello M, et al. An electronic patient-reported outcome mobile app for data collection in type A hemophilia: design and usability study. JMIR Formative Research. 2021;5(12). doi:10.2196/25071
11. Hernar I, Graue M, Richards D, et al. Electronic capturing of patient-reported outcome measures on a touchscreen computer in clinical diabetes practice (the DIAPROM trial): a feasibility study. Pilot and Feasibility Stud. 2019;5(1). doi:10.1186/s40814-019-0419-4
12. Kyte D, Anderson N, Auti R, et al. Development of an electronic patient-reported outcome measure (EPROM) system to aid the management of patients with advanced chronic kidney disease. J Patient Rep Outcomes. 2020;4(1). doi:https://doi.org/10.1186/s41687-020-00223-8 10.1186/s41687-020-00223-8
13. Gabbard J, McLouth CJ, Brenes G, et al. Rapid electronic capturing of patient-reported outcome measures in older adults with end-stage renal disease: a feasibility study. Am J Hosp Palliat Care. 2020;38(5):432-440. doi:10.1177/1049909120954805
14. Radhakrishnan K, Julien C, Baranowski T, et al. Feasibility of a sensor-controlled digital game for heart failure self-management: randomized controlled trial. JMIR Serious Games. 2021;9(4). doi:10.2196/29044
15. Koumakis L, Schera F, Parker H, et al. Fostering palliative care through digital intervention: a platform for adult patients with hematologic malignancies. Front Digit Health. 2021;3. doi:10.3389/fdgth.2021.730722
16. Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors: a role for patient-reported outcome measures and health informatics. Acta Oncologica. 2015;54(5):600-608. doi:10.3109/0284186x.2014.995778
17. Abdelazeem B, Abbas KS, Amin MA, et al. The effectiveness of incentives for research participation: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2022;17(4). doi:10.1371/journal.pone.0267534
18. Brueton VC, Tierney JF, Stenning S, et al. Strategies to improve retention in randomized trials: a Cochrane systematic review and meta-analysis. BMJ Open. 2014;4(2). doi:10.1136/bmjopen-2013-003821
19. Christofides E, Stroud K, Tullis DE, O’Doherty KC. Improving dissemination of study results: perspectives of individuals with cystic fibrosis. Res Ethics. 2019;15(3-4):1-14. doi:10.1177/1747016119869847

