ISPOR Conferences and Events
ISPOR Conferences and Events

HEOR: A Transformative Force for Whole Health
Be there next month when experts from all areas of healthcare gather at the Georgia World Congress Center, Atlanta, GA, USA, for ISPOR 2024, the leading global conference for health economics and outcomes research (HEOR). Be sure to add the Digital Conference Pass to your registration to access recordings of nearly all educational sessions.
Mark your calendar for these plenary sessions:
Monday, May 6 | 8:30AM EDT
Advancing Whole Health: How do We Know When We’re Succeeding?
Tuesday, May 7 | 8:30AM EDT
Missing Link for HEOR: A Path Forward for HEOR Data Integration
Wednesday, May 8 | 11:30AM EDT
AI Enabling Whole Health: Opportunities and Challenges for HEOR and HTA
The following is a sampling of sessions and hot topics from across the HEOR spectrum:
Health Policy and Regulatory
Medicare Drug Price Negotiation: Lessons from the First 10 Drugs Selected [Spotlight session]
Economic Evaluation
How to Adjust Economic Models for Health Equity in the Conduct of Generalized Cost-Effectiveness Analysis [Spotlight session]
Methodological and Statistical Research
Unlocking the Potential of Open Source Models: Strategies to Navigate Barriers in Development and Adoption [Forum]
Patient-Centered Research
Including the ISPOR Patient-Centered Research Summit 2024 [co-located at ISPOR 2024 | May 5]
Real-World Evidence
Revolutionizing Regulatory Pathways: Unleashing the Power of Real-World Evidence, Adaptive Trials, and Synergistic Collaboration for Expedited FDA Device Approval, Breakthrough Designation, and CMS Reimbursement [Panel session]
Pre-conference Short Courses
A full day of short courses will be held on May 5. The ISPOR Short Course Program is designed to enhance knowledge and techniques in core HEOR topics as well as emerging trends in the field. Taught by expert faculty, courses span across 7 topical tracks and range in skill level from introductory to experienced.
More at www.ispor.org/ISPOR2024
Join the conversation on Twitter #ISPORAnnual

A co-located event at ISPOR 2024, this year’s Summit theme is “Advancing Patient-Centered Research.” The half-day Summit promises to be an enriching and informative gathering of experts, researchers, and practitioners in the field, where we will explore the latest advancements, emerging standards, and breakthroughs in patient-centered research. Attendees can engage in dynamic discussions on strategies, regulatory policies, and methods that enhance the influence of patient involvement in generating evidence and shaping healthcare decisions.
Sessions include:
The Power of Patient Voices: Elevating Patient-Centered Outcomes in Research
Making a Difference: Identifying Best Practices to Measure the Impact of Patient Engagement
Patient-Centered Methods: Accomplishments, Innovations, and the Future
Advancing Patient-Centered Research: Seizing Opportunities and Addressing Challenges
Learn more about the Summit and register here.
Call for Abstracts!

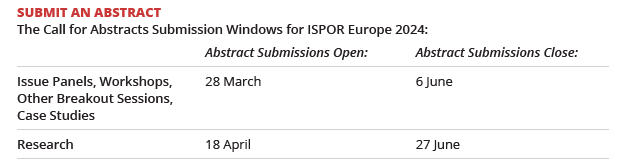
Mark your calendars for ISPOR Europe 2024, the leading European conference for health economics and outcomes research (HEOR) this 17-20 November! Network with your peers, HEOR experts, and thought leaders. Submit your issue panel, workshop, other breakout session or case study abstract for an opportunity to interact and discuss your innovative experiences in outcomes research with a global audience. Research submissions open 18 April. Submit today!
Details at www.ispor.org/ISPOREurope2024
Join the conversation on social media using the official conference hashtag #ISPOREurope
Get in front of your target audience and ensure your company is included in the conference Exhibitor Guide! Contact sales@ispor.org.

A co-located event at ISPOR Europe 2024, the 17 November Summit will the cover latest developments in use of real-world evidence (RWE) across the regulatory health technology assessment-payer decision-making continuum with a focus on methods, data transportability, and infrastructure. Major topics to be covered include causal inference and external control arms for comparative effectiveness analyses, the hierarchy of RWE studies, and the role of patient registries. The use of RWE in Joint Clinical Assessment of the EU HTA regulation will be explored, with insights from the cross-border collaborations on pricing and reimbursement in the EU countries. In addition, the feasibility of HTA reassessment post market entry will be considered, drawing from lessons learned from US Medicare Drug Price Negotiation.
ISPOR Education

The ISPOR Education Center provides instant access to HEOR education with on-demand programs delivered through a personalized, powerful, and flexible learning platform. Working at their own time and pace, individuals can drive their professional development by growing their knowledge and skills with topical, relevant, and innovative course curricula.
Patient-Focused Medical-Product Development
After the completion of this course, participants will be able to:
- Understand patient engagement in medical-product research and its development by defining the terms, providing historical context, and illustrating its significance throughout ISPOR’s HEOR taxonomy.
- Use tools to plan and implement meaningful patient-engagement activities in their respective areas of research
(eg, clinical development, epidemiology, health economics, real-world evidence, etc). - Provide solutions for addressing challenges in implementing patient engagement in a clinical research environment.
- Describe real-world examples of patient-researcher partnerships, best practices, and practical solutions to challenges.
View the featured courses, topics covered, and the growing list of courses available at www.ispor.org/EducationCenter

The HEOR Learning Lab™ is ISPOR’s educational resource for professionals who work or have an interest in the field of HEOR. The HEOR Learning Lab provides unlimited on-demand, educational video content to facilitate learning and innovative approaches in the field from the leading global organization in HEOR. Easily searchable content is focused on the most topical themes impacting the field, including real-world evidence, patient-centered research, digital health, artificial intelligence and machine learning, health technology assessment, economic methods, healthcare financing, access and policy, learning healthcare systems, and much more. More than 500 sessions from Society conferences, summits, and other seminal events are currently available on demand on the platform. Newly added content includes sessions from ISPOR Europe 2023!
Visit the HEOR Learning Lab at www.ispor.org/LearningLabWelcome

ISPOR short courses are designed to enhance knowledge and techniques in core health economics and outcomes research (HEOR) topics as well as emerging trends in the field. Short courses offer 4 or 8 hours of premium scientific education and a digital course book. Active attendee participation combined with our expert faculty creates an immersive and impactful virtual learning experience. Short courses are not recorded and are only available during the live broadcast.
Upcoming ISPOR Short Courses include:
April 10-11 | 10:00AM – 12:00PM EDT (Virtual)
Budget Impact Analysis I: A 6-Step Approach
What you will learn in this introductory-level course:
- Understand 6 steps needed to complete a budget impact analysis.
- How to distinguish between static and dynamic budget impact models.
- Ways to design a study to estimate the budget impact of a new healthcare intervention using the 6-step approach.
April 17-18 | 10:00AM – 12:00PM EDT (Virtual)
Learning and Applying Discrete Event Simulation
What you will learn in this introductory-level course:
- Ways to explain the steps and design choices necessary for developing discrete event simulations.
- How to distinguish between alternative modeling approaches for implementing competing events in discrete event simulations depending on the type of evidence to be used.
- How to distinguish between different types of uncertainty and variation in discrete event simulations.
- Methods to develop and run a basic discrete event simulation using the simmer package in R.
June 5-6 | 10:00AM – 12:00PM EDT (Virtual)
Digital Real-World Evidence Generation Approaches in Rare Diseases and Oncology
What you will learn in this introductory-level course:
- Gain a clear understanding of important study documents.
- Techniques to draft study protocol and informed consent.
- An understanding of what drives an ethical approval strategy and the drivers of an effective information
governance strategy. - How to build an effective data capture strategy, comprising PROMs and the capture of other types of data.
- Methods to incorporate collaborative tools into study design.
View all short courses available during ISPOR 2024
Learn more about the www.ispor.org/shortcourses

Upcoming webinars include:
April 10 | 11:00AM – 12:00PM EDT
Revolutionizing Clinical Trials: Harnessing Real-World Evidence to Drive Diversity and Clinical Implementation
By completing this webinar, you will:
- Explore the transformative potential of real-world evidence (RWE) in enhancing clinical trial design and execution.
- Discuss cutting-edge technologies shaping the future of clinical research, from Natural Language Processing to AI-driven analytics.
- Identify practical strategies for integrating RWE and technology to optimize trial efficiency and outcomes.
May 20 | 10:00AM – 11:00AM EDT
Revolutionizing Systematic Reviews: Harnessing the Power of AIBy completing this webinar, you will:
- Gain an understanding of the current state of AI adoption in systematic reviews.
- Benefit from live demonstrations of several pioneering AI-driven platforms designed to optimize and expedite systematic review processes.
- Acquire practical insights into the applications of AI, understanding how these platforms enhance efficiency, accuracy, and overall effectiveness in systematic reviews.
May 22 | 10:00AM – 11:00AM EDT
The Global Socioeconomic Impact of Rare Diseases: A Call for Action
By completing this webinar, you will:
- Gain an understanding of studies conducted in high-income countries, and the impact of data scarcity in lower- and middle-income countries on the socioeconomic estimates.
- Understand the consequences of low investment in rare diseases on the overall burden.
- Learn to gauge the value of investing in diagnosis and early interventions.
May 28 | 10:00AM – 11:00AM EDT
The Role of RWE for Devices and Diagnostic Market Access in Europe
By completing this webinar, you will:
- Understand country-specific key decision pathways for medical devices and diagnostics reimbursement and learn which of these requirements can be satisfied by real-world evidence (RWE).
- Be introduced to specific examples on how RWE was used to satisfy country-specific HTA hurdles.
- Understand the strengths and limitations of real-world data in the context of utility in reimbursement decision making.
May 29 | 10:00AM – 11:00AM EDT
Are Clinical Outcome Assessments Sufficiently Valued?
By completing this webinar, you will:
- Increase your awareness of the complexity in developing clinical outcomes assessment (COA) strategies to meet different stakeholder requirements.
- Develop a greater understanding of the value of COA data from diverse perspectives and identify synergies across the value of COA data among various stakeholders.
- Gain a greater awareness of various initiatives in development to enhance the value of COA data.
June 11 | 12:00PM – 1:00PM EDT
Overcoming the Barriers of Open-Source Modeling
By completing this webinar, you will:
- Gain an understanding of why the uptake of open-source modeling (OSM) has been limited.
- Identify the barriers to the development and use of OSM and strategies to overcome these barriers according to different stakeholders.
- Determine the most promising strategies for optimizing the use of OSM and the next steps in implementation.
View upcoming and on-demand ISPOR Webinars: www.ispor.org/webinars

The HEOR Solutions Center is an online business community that connects health economics and outcomes research (HEOR) professionals with the solutions they need for their businesses and organizations. Connect with leading health research consulting firms, contract research organizations, data management providers, digital innovators, and more. Find the right solutions to meet your business needs. Learn more about the HEOR Solutions Center at www.ispor.org/HEORSolutionsCenter.
Interested in becoming an integral part of ISPOR’s online business community? For more information on joining the HEOR Solutions Center, contact sales@ispor.org or download the HEOR Solutions Center Product Information here.

