The Evaluation of Pivotal Trials for Advanced Therapies From Regulatory and Health Technology Assessment Perspectives in Europe
Tingting Qiu, PhD, Aix-Marseille University, Marseille, France; Monique Dabbous, PhD, Aix-Marseille University, Marseille, France; Borislav Borissov, PhD, Prescripta, Sofia, Bulgaria
The novelty and complexity of advanced therapies have raised barriers in their development as well as challenges at regulatory and market access levels. Clinical trials for advanced therapies are typically open-label, single-arm trials with small sample sizes and short follow-up durations. Inconsistencies were identified between regulators and payers in terms of acceptance of such short-term noncomparative clinical evidence for advanced therapies. The uncertainty relating to the evidence for these therapies should be sufficiently addressed to satisfy the expectations of both parties at the time of launch.
Pivotal studies for approved advanced therapies have been evaluated by health technology assessment (HTA) bodies in major European countries with an aim to enlighten the design of future advanced therapy trials.
Enhanced interactions between regulators and HTA bodies are needed to reach an agreement on the evidence requirements for not only pivotal clinical trials, but also for postmarketing evidence-generation requirements to be fulfilled.
Challenges in the Clinical Trials for Advanced Therapy Medicinal Products
Advanced therapy medicinal products (ATMPs) hold great potential to transform the conventional paradigm of disease management by targeting the underlying causes of the diseases. ATMPs represent significant promises for rare genetic disorders lacking alternative treatment. Due to the unique and complex nature of ATMPs, they are associated with challenges at the manufacturing and clinical development levels as well as significant hurdles to achieving market access. In particular, conducting conventional randomized clinical trials to collect reliable and robust evidence could be more challenging for ATMPs than for traditional drugs. As most ATMPs target rare disorders,1 they will face the same challenges associated with orphan drugs. The small target population of ATMPs leads to difficulties with regards to patient recruitment and conducting head-to-head studies. Most rare genetic diseases have no curative treatments, which requires patients to rely on symptomatic treatments and results in great variations in the standard of care.2 Blinding patients and/or physicians seems impractical due to novel methods of administration and the unique safety profiles of ATMPs. In addition, patients have expressed hesitations to participate in blinded clinical trials with a placebo or less effective standard of care when an active therapy, especially with a potential for cure, may be available.3 This has also been cited as a reason for failure in patient recruitment for several COVID-19 studies as active treatments could be made available through other paths.4 Furthermore, ATMP clinical trials are required to be conducted only in authorized centers with adequate infrastructures for the administration and management of ATMPs. This increases patient burden of traveling to trial sites and may also result in potential selection bias towards patients able to endure traveling.5
Differences Between Regulators and Payers in the Evidence Requirements for ATMPs
Regulation EC No 1394/2007 on ATMPs introduced a more flexible approach for the assessment of quality, safety, and efficacy evidence of an ATMP taking into consideration the novelty, complexity, and technical specificity of ATMPs. Moreover, ATMPs represent therapeutic advantages in addressing the unmet clinical needs for devasting conditions; thus ATMPs are highly likely to be eligible for expedited pathways, such as conditional marketing authorization and Priority Medicine designation.6 This translates into an increased approval rate of ATMP despite nonconventional studies, such as single-arm trials and very small sample size studies in ultrarare conditions (Table 1). Regulators have shown a willingness to grant access to this new class of pharmaceuticals based on favorable benefit–risk balances despite the magnitude of the benefits remaining difficult to quantify due to short-duration single-arm studies and the frequent use of surrogate outcomes.
Table. Study methodology of pivotal studies for ATMPs submitted for market authorization and HTA.
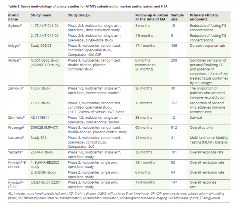
In contrast to regulators’ enthusiasm to facilitate the timely marketing authorization based on limited evidence, HTA bodies hold more conservative attitudes towards ATMPs. HTA bodies concluded that limited clinical evidence increased the uncertainties surrounding the curative potential, the magnitude and the durability of clinical benefits, and the potential unfavorable effects in the long run. Consequently, the determination of value as well as justification of the high treatment cost relative to its value seemed inconclusive, rendering payers reluctant or hesitant to reimburse ATMPs within constrained budgets.7 Furthermore, there are disparities in evidence requirements for HTAs; for example, the acceptance of indirect comparisons and the methods for heterogeneity adjustments may differ across HTA bodies.
The methodology of clinical trials for ATMPs was the primary cause for reserved opinions of HTA bodies about ATMP. It is interesting to analyze how approved ATMPs have been assessed in order to identify where efforts could be implemented to increase the chances of positive recommendations by HTA bodies for future ATMPs.
"Patients have expressed hesitations to participate in blinded clinical trials with a placebo or less effective standard of care when an active therapy, especially with a potential for cure, may be available."
Uncertainties in the Pivotal Evidence for Approved ATMPs
Based on the HTA outcomes for approved ATMPs in selected European countries (England, Scotland, France, Germany, and Sweden), the key limitations of clinical evidence for ATMPs were identified to be due to study design, study population, study endpoints, statistical analysis methods, confounding factors, and indirect comparisons.
The design of pivotal studies for ATMPs was the most frequently criticized by HTA bodies, including the single-arm trial design, small sample size, short follow-up duration, high dropout rate, and discordance between the dosage regimen in the clinical trials and the marketing authorization. Regarding comparative studies, limitation was associated with the comparator selection, which was either not considered as the standard of care or it was no longer applied in clinical practice, making it difficult for the HTA body to appreciate the ATMP value.
The main issues related to the study population were discrepancies between the patients recruited in the clinical trials and the marketing authorization label, the exclusion of patients with a higher disease severity in the trial, and the lack of representation of the study population to the treatment-eligible population.
The limitations of the study endpoints were mainly the use of surrogate endpoints as primary endpoints, the clinical relevance and the validity of primary endpoints in the real-world setting, analysis on a posthoc basis but not prespecified, and the reliability of endpoints due to the intertest variability.
The statistical analysis methodologies presented limitations with regards to the following: incomplete information on the imputation procedure for missing values; unreliable survival extrapolation based on short-term clinical evidence; uncertainty in the estimation of the long-term survival using the Kaplan-Meier method; and potential overestimation of treatment benefits resulted from full analysis set analysis rather than intention to treat analysis.
The presence of confounding factors that may bias the treatment benefits estimated could not be ruled out in several cases. This mainly included the impact of dietary regime on treatment effect, long waiting periods and bridging treatment prior to randomization, imbalance in patient characteristics and withdrawal rates between 2 comparison groups, and the questionable appropriateness of pooling data from heterogenous studies.
The challenges in the transferability and generalizability of clinical data were criticized, mainly around the disparity between the treatment pathways adopted in the study and real-world clinical practice and the extrapolation of short-term clinical evidence for long-term treatment benefits.
Indirect comparison was generally derived using data from systematic reviews and meta-analyses, historical comparisons, patient registries, or network databases. The main limitations of indirect comparisons were indicated in the heterogeneity of patient characteristics, differences in the outcomes investigated, potential biases due to uncontrolled confounding factors, and the difficulties to draw firm conclusions regarding relative effectiveness comparing with historical observational studies of flawed methodology. The inherent limitations for studies utilizing indirect comparison included small patient populations, incomplete information about baseline patient characteristics, inability to trace retrospective studies, and the potential selection bias for studies included in meta-analyses.
"Enhanced interactions between regulators and HTA bodies are needed to reach an agreement on the evidence requirements for not only pivotal clinical trials, but also for postmarketing evidence-generation requirements to be fulfilled."
Future Insights for Comprehensive Evidence Collection
Considering the substantial number of potentially transformative ATMP marketing authorization applications in the next years, regulators realized the urgency to provide more clarification on regulatory requirements in order to support ATMP developers in early clinical trial design. Earlier this year, the US Food and Drug Administration released a series of guidelines on the recommendations for specific diseases with a large number of products in development, such as guidance on gene therapy for hemophilia, retinal disorders, and Sanfilippo Syndrome. However, there are no specific HTA guidelines for ATMP development. Because HTA bodies offer a limited number of early advices to help developers in the clinical development plan preparation, HTA guidelines providing clearly defined requirements on the study methodology and economic assessment would be embraced by developers to better prepare the HTA dossiers.
Moreover, both regulators and HTA bodies emphasized the need for post-launch evidence generation to bridge the evidence gap with initial assessments of ATMPs. Regulators expect post-launch evidence to confirm positive benefit–risk balances as claimed, while HTA bodies will utilize it for subsequent price negotiations and decisions on the continuation or termination of performance-based payments. Post-launch follow-up studies are conducted for almost all approved ATMPs to assess the long-term effectiveness and safety. However, study designs of these post-launch studies generally have resembled those of pre-approval studies in terms of strict inclusion criteria and the small number of patients to be enrolled.8 This has raised skepticism regarding whether such postlaunch studies will be sufficient for HTA bodies to provide confirmatory evidence on the actual treatment benefits of ATMPs in real-world scenarios.
Next Steps
Enhanced interactions between regulators and HTA bodies are needed to reach an agreement on the evidence requirements for not only pivotal clinical trials, but also for postmarketing evidence-generation requirements to be fulfilled. Additionally, further efforts are needed in standardizing the methodology to collect, report, and analyze the data from postmarketing observational studies in order to mitigate inherent limitations. Centralized disease or product registries, instead of numerous protocols from individual companies developing ATMPs with similar mechanisms of action, will be valuable to streamline the evidence collection and improve the quality of postmarket data. •
References
1. Lapteva L, Vatsan R, Purohit-Sheth T. Regenerative medicine therapies for rare diseases. Transl Sci Rare Dis. 2018;3:121-132.
2. Abrahamyan L, Feldman BM, Tomlinson G, et al. Alternative designs for clinical trials in rare diseases. Am J Med Genet C Semin Med Genet. 2016;172:313-331.
3. Gaasterland CMW, van der Weide MCJ, du Prie-Olthof MJ, et al. The patient’s view on rare disease trial design-a qualitative study. Orphanet J Rare Dis. 2019;14:31.
4. Joseph A. Efforts to beat back the coronavirus are critical. They’re also making clinical trials harder. 2020. https://www.statnews.com/2020/05/05/efforts-to-beat-back-the-coronavirus-are-critical-theyre-also-making-clinical-trials-harder/
5. The full reference is: White W. A rare disease patient/caregiver perspective on fair pricing and access to gene-based therapies. Gene Therapy. 2020;27:474–481.
6. European Union. Regulation (EC) No. 1394/2007 of the European Parliament and of the Council of 13 November 2007. On advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Official J Eur Union. 2007;(L324)121.
7. Ermisch M, Bucsics A, Vella Bonanno P, et al. Payers’ views of the changes arising through the possible adoption of adaptive pathways. Front Pharmacol. 2016;7:305.
8. Fritsche E, Elsallab M, Schaden M, et al. Post-marketing safety and efficacy surveillance of cell and gene therapies in the EU: A critical review. Cell Gene Ther Insights. 2019;5:1505-1521.
Explore Related HEOR by Topic

