How Health Technology Assessment Supports Universal Health Coverage in the Asia Pacific
Robert Selby, MBA, Director, Global Networks - Asia Pacific and Latin America, ISPOR, USA
While the COVID-19 pandemic has greatly disrupted health systems in the Asia Pacific region and globally, health technology assessment (HTA) has remained a pillar for healthcare decision making over the long haul. Recently, HTA’s role in informing countries’ policies around the prioritization and allocation of critically needed resources in hospitals and generating rapid reviews of technologies for emergency use has been essential during the pandemic. But even before the pandemic hit, many countries in the region were already on the long journey toward adopting or strengthening universal health coverage frameworks in their health systems. Throughout that process, HTA has been an indispensable tool for supporting the development of health benefits packages (HBP) within the UHC frameworks, by providing evidence-based recommendations surrounding both the clinical efficacy and the cost-effectiveness, financial, and society-wide implications of interventions. In this way, HTA has helped policy makers make practical reimbursement and coverage decisions that balance the conflicting pressures of providing citizens with broader access to comprehensive care while also maintaining fiscal sustainability for the health system.
COVID-19: Disruptor and Accelerator in the Healthcare Landscape
To better understand how the COVID-19 pandemic has impacted HTA bodies in the Asia Pacific region, the ISPOR HTA Council fielded a survey among its HTA roundtable delegates asking the question, “What are the key challenges and opportunities that have surfaced as a result of COVID-19 from the regional perspective?” Figure 1 shows the specific challenges (orange tiles) and opportunities (blue tiles) raised by the respondents. From the delegates’ perspective, the challenges clearly outweigh the opportunities, and a major question was raised: “What is the role of HTA agencies in advancing accurate, evidence-based information to the public?” According to Dr Li Ying (Grace) Huang, Director of the Division of the Health Technology Assessment, Center for Drug Evaluation, Taiwan, “The importance of fair and transparent decision making and the consideration of patients and public preferences around the availability of high-cost medicines are likely to become increasingly prominent. HTA is well-placed to consider the value proposition from a broader social and health system perspective rather than solely from a patient perspective and has become an essential priority-setting process that adopts the principles of procedural justice and is used to inform policy and access decisions.” It seems that even under the current pandemic situation, HTA’s role in providing objective and unbiased information remains crucial to ensuring that decisions are taken in a transparent and evidence-based manner.
Figure 1. Specific challenges (orange tiles) and opportunities (blue tiles) raised by the respondents

HTA and UHC: Supporting Patient Access Across the Asia Pacific Region
In Taiwan, continuous reform under the National Health Insurance program has resulted in the expansion of health insurance coverage to over 99% of the 23.4 million population, while keeping premiums and copayments low for citizens. When it comes to the role of HTA in evidence-based policy-making in Taiwan, HTA’s stated goal is to support the health authority to maximize the public health benefits. Specifically, the HTA team under the Center for Drug Evaluation primarily assesses the clinical comparative effectiveness and economic evaluation of new drugs and medical devices to support the decision making of the National Health Insurance program. A significant feature of Taiwan’s process is the mechanism for consideration of multistakeholder and multidisciplinary perspectives. Taiwan’s HTA process follows a well-rounded deliberative framework that covers 4 pillars: (1) budget and financial impact,
(2) human health (comparative effectiveness and safety), (3) cost-effectiveness, and (4) medical ethics (unmet medical need). This comprehensive approach allows for broader consideration of the value of health technologies.
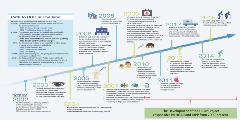
In Thailand, there are 3 different insurance schemes covering different segments of the population. Together, they cover a combined 99% of the population of 66 million. While 2 of the 3 programs include a closed ended annual budget, the benefits package has consistently expanded through the universal health coverage (UHC) framework since 2002 (Figure 2). HTA plays a key role in determining which technologies receive approval and coverage, considering the dimensions of cost-effectiveness, budget impact, and feasibility. Approved pharmaceutical products are included in the National List of Essential Medicines and non-pharmaceutical products are included in the UHC benefits package scheme. The recently updated 2020 HTA guidelines for Thailand have included new sections that provide further clarification on issues such as feasibility studies of the use of health technology, the use of real-world evidence in HTA, and the evaluation of health economic value for health technology in biosimilar products, codependent technologies, health promotion measures, complex health intervention measures, and the measures for rare diseases (Suchonwanich, N. Virtual ISPOR Asia Pacific 2020 Plenary session, Sep 2020).
Figure 2. Expanding benefits package under the Universal Coverage Scheme in Thailand
In South Korea, patient access to therapies is expanding through key programs such as financial-based risk-sharing agreements, which have experienced a marked increase in prevalence (there were 11 risk-sharing agreements in 2016 compared to 35 in 2020). A coverage expansion policy by the National Health Insurance (NHI) program has also led to an increase in the overall coverage rates for interventions, where provisions are made for selective or preliminary coverage of novel therapies with subsequent evidentiary development over a 3- to 5-year timeline. These arrangements are accompanied by tiered rates of coverage ranging from 50%, 20%, and 10%. There also has been an expansion of indications for some procedures, such as magnetic resonance imaging and ultrasonography. The program encompasses 170 items including transcatheter aortic valve implantation and navigation procedures for surgeries. To speed up the assessment process, a parallel review for medical device-related procedures has been instituted where simultaneous review is undertaken by the Ministry of Food and Drug Safety (safety and efficacy), National Evidence-Based Healthcare Collaborating Agency (nHTA, or HTA of medical devices and procedures), and the Health Insurance Review & Assessment Service (reviewing existing comparators) to streamline the approval of technologies. The combination of these new reforms and approaches has made a significant impact on patient access; for example, in vitro diagnostics are now subject to faster uptake with nHTA suspended for 1 to 5 years, where they are managed with preliminary coverage and ongoing real-world evidence assessment.
In India, the Ayushman Bharat UHC scheme of the Government of India has expanded its coverage to more than the initial 40% of the population through its comprehensive primary care centers, along with the Pradhan Mantri Jan Arogya Yojana (PMJAY) health insurance scheme covering secondary and tertiary healthcare centers. The institutionalization of HTA in the country since 2017, aided by capacity building for evidence-based policy making, is envisioned to play a crucial role towards strengthening UHC in India. The cost evidence from the Nationwide Costing Initiative of the HTA body in India has guided the price setting of PMJAY health benefit packages during its revision. Within the Indian context, 3 dimensions to consider when moving towards UHC include equitable access to health services, provision of good quality services to maximum people, and reduction in financial risk.
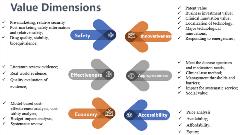
In mainland China, HTA systematically informs revisions to the essential medicines list, which is the list of therapies covered by the national health insurance program. Established committees regularly evaluate and adjust the essential medicines list every 3 years, prioritizing drugs with clear evidence of effectiveness, safety, and significant cost-effectiveness. In conducting the HTA, the following dimensions are considered: safety, effectiveness considering the available evidence, economy, innovativeness, appropriateness, and accessibility. The consideration and judgment of value is also becoming broader, with key value dimensions going beyond traditional clinical and economic value to include social value (Figure 3). Multicriteria decision analysis (MCDA) is also employed as a comprehensive value judgment tool to capture preferences and perspectives from a wide range of healthcare stakeholders including patients, physician and pharmacist groups, hospital and medical service providers, healthcare payers, and government. Based upon the review results, there are several potential positive outcomes for recommendation, including acceptance into the reimbursed list of essential medicines; conditional acceptance that may be deemed appropriate for certain subgroups, indications, dosages or routes of administration; or even deployment in pilot implementation. These new avenues for consideration and approval all provide an added measure of versality and flexibility and expand new pathways for patient access.
Figure 3. Value dimensions in China