Accelerating Patient Access to Next-Generation Sequencing in Oncology: A Plan of Action
Tim Wilsdon, MSc, Charles River Associates, London, England, United Kingdom; Denis Horgan, PhD, The European Alliance for Personalised Medicine, Brussels, Belgium; and Marlene Akkermans, MSc, MSD Innovation & Development GmbH, Zurich, Switzerland

The benefits of NGS in oncology
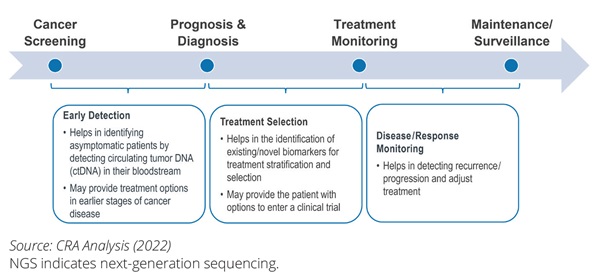
Precision medicine requires that patients are tested for genetic alterations (known as targets) to diagnose diseases and consider treatment options. NGS is a form of DNA sequencing that can examine millions of DNA molecules simultaneously, offering benefits for clinical trial enrollment or patient treatments.1 As illustrated in Figure 1, NGS has multiple applications for patients along the patient journey.
NGS testing has already been recommended in clinical guidelines for use in indications such as non-small cell lung cancer (NSCLC), cholangiocarcinoma, and prostate and ovarian cancers. There are existing recommendations on the routine use of NGS from the European Society for Medical Oncology (ESMO),2 as well as by the American Society of Clinical Oncology (ASCO). An updated provisional clinical opinion from ASCOin April 2022 recommends that patients with metastatic/ advanced cancer should undergo genomic sequencing if one or more specific genomic alterations have regulatory approval as biomarkers to guide the use of or exclusion from certain treatments for their disease.3 Furthermore, multigene panel- based assays (many of which are based on NGS technology) should be used if more than one biomarker-linked therapy is approved for the patient’s disease.
Figure 1. NGS delivers benefits to oncology patients across their patient journeys
The benefits of NGS extend beyond the direct clinical benefits to patients. NGS may also deliver value to healthcare systems and payers and has a broader societal and economic impact as illustrated in Figure 2. The primary benefits of NGS can be the breadth of diagnostic data that it unlocks and the ability to provide a test outcome for multiple genetic and genomic mutations at once, allowing for both faster and greater diagnostic accuracy that ultimately helps guide relevant patients to the most suitable targeted therapies.
Figure 2. An overview of the benefits of NGS testing to patients, healthcare systems, payers, and society as a whole.
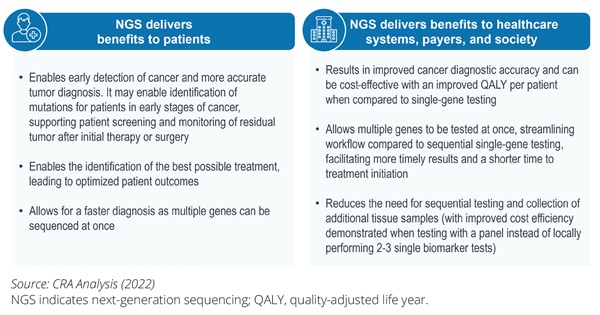
For example, in care for patients with cancer, any given tumor may be driven by different mutations. When traditional single-biomarker testing is used in patient diagnosis, multiple diagnostic tests may need to be performed sequentially. This may lead to a longer time to diagnosis and unavailability of tissue to perform these sequential tests, thus resulting in higher costs. Multiple gene alterations can be interrogated in one test when using NGS, resulting in less tissue being needed than sequential approaches to testing and the results from the dozens and hundreds of potential DNA targets obtained from one test.1 The benefits may go beyond the clinical setting as patients with knowledge of their genetic and genomic profiles may make more informed decisions (eg, treatment adherence or preventive measures). Finally, the patient’s increased knowledge about the disease and the available treatment options4 has the potential to increase the quality of life and reduce the mental burden of disease, resulting in increased well-being and a sense of personal control, thereby creating more patient-centered cancer care by aligning patient preferences with health system options.5 At the same time, we should recognize that some uncertainties regarding the use of NGS remain. Further research may be required to identify the broader benefit of NGS across different treatment stages, indications, and resource settings. In addition, there may be a need for further evidence on linkage between upfront NGS testing and patient outcomes after the NGS-directed therapy.
"Patient's increased knowledge about available treatment options has the potential to create more patient-centered cancer care by aligning patient preferences with health system options."
NGS can deliver value to healthcare systems and in the long-term, help to improve sustainability. Multiple gene testing may help reduce the number of tests required. It provides relatively fast and accurate diagnosis even in cases when biopsy samples are limited.6 This means that, in the future, NGS testing could require less effort and fewer resources expended in pathology laboratories for both testing and analysis, which could bring cost savings to the healthcare system. Most importantly, through NGS, the diagnosis of patients could be improved or accelerated, resulting in appropriate treatment or clinical trial options being identified earlier, which in turn may prevent the use of ineffective treatment and avoid societal cost. NGS has the potential to reduce the number needed to treat and thereby improve healthcare sustainability.
The data on the cost-effectiveness of NGS in particular tumor types and in particular countries and regions are growing over time. Most of these studies are based on US experience but recent studies from European countries and from Latin America show the clinical and economic benefits of NGS in multiple solid- tumor cancers, NSCLC, and early stage breast cancer.7 Because there are relatively few studies on health outcomes that provide data on cost-effectiveness of NGS and/or that report formal cost per quality-adjusted life year (QALY) analysis, more research may be needed in this area.
The key barriers hindering access to NGS testing in oncology
Oncology patients are tested in primary or secondary care with a blood or biopsy sample sent to the laboratory. NGS testing is then conducted through in-house laboratory testing or by sending the sample to a central laboratory for testing and reporting.
In cases where an NGS test cannot be processed in-house but is sent to a different laboratory either in a center of excellence or in a testing facility abroad, this may lead to an increase in processing time and reporting of the test result. Regardless of where the test is performed (in-house, in a center of excellence, or abroad), test results are reported back to the patient’s oncologist and indicate whether gene mutations have been found, if a suitable treatment has been identified, and whether the patient could be eligible for a clinical trial.
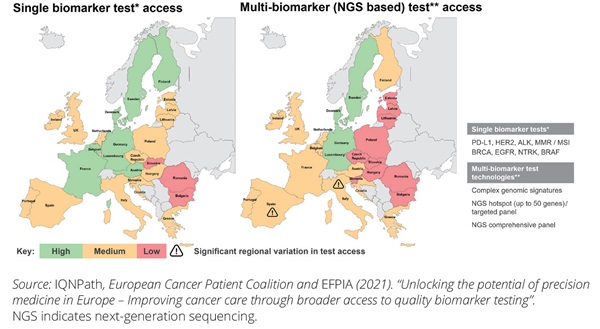
The degree of access to NGS testing for oncology varies geographically as illustrated in Figure 3. The left-hand side of the map focuses on non-NGS, (ie, single-biomarker testing), while the right-hand side represents NGS (ie, showing less access with NGS compared to single-biomarker). The index used demonstrates that access is a multifactorial issue. Additionally, it includes a measure of laboratory access (including regional availability of diagnostic labs and the efficiency of referral pathways), the availability of NGS testing, integration of testing into clinical practice, and NGS test reimbursement. As of today, there are wide disparities within and between countries. The overall infrastructure and access environment for NGS is most advanced in the United States, where NGS testing is supported by ASCO guidelines and reimbursement is covered via Medicare and, for several indications, via private insurance. NGS testing in the United States is available in centers of excellence and through test providers with central labs.
Figure 3. There is inequitable patient access to NGS testing within and across countries
On May 2, 2022, ISPOR organized an expert panel and open webinar to discuss the barriers to NGS access and then identify potential solutions to resolve these access barriers. After first testing the range of barriers with the webinar audience, the panelists discussed the extent to which these could be prioritized. There was general agreement among the panelists that NGS faces a set of interlocking challenges (Figure 4).
Figure 4. A range of significant barriers are impeding the broader uptake of NGS in oncology clinical practice
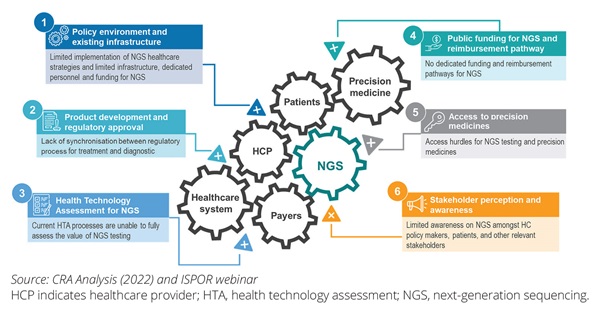
The discussion during the webinar focused on 3 of these as key barriers.
• The overall NGS policy environment and existing infrastructure:
The diagnostic test infrastructure plays a key role in a country’s uptake of NGS testing. A national (or supranational in the case of the European Union) policy on genomic testing could be an important enabler to design the national infrastructure for NGS testing. Several countries, including France, Germany, the United Kingdom, and the United States, have introduced national policies designed to increase investments in genomics and increase diagnostic capacity. For example, there have been initiatives in the United Kingdom (the 100,000 Genomes Project)8 and in Germany (genomDE)9 to establish a national genome initiative to coordinate numerous existing programs and to improve the opportunities for care and research in the fields of cancer and rare diseases. In the European Union, these have been consolidated into the 1+ million Genomes Initiative, which has the aim of collecting large amounts of genomic data for research, prevention, and personalized medicine purposes.10 Alongside the activities directly aimed at genomic testing, the Cancer Diagnostic and Treatment for All flagship initiative (part of Europe’s Beating Cancer Plan) encourages member states to develop national plans with a focus on patient access and improving diagnosis.11 It will be important to turn these plans into action at the national level, addressing the divergent levels of access to high-quality oncology biomarker testing across Europe. Today, there is significant variation with Southern and Central European countries as well as the Baltic countries lagging behind Northern and Western European countries (Figure 3) in terms of the infrastructure and funding for multigene or NGS biomarker testing.12 Significant work is needed at the national level for a functioning governance framework that facilitates access to NGS testing for all patients with cancer.
• Public funding regime and health technology assessment (HTA) for NGS testing:
A key barrier to NGS access is the approach to funding, including the decision making on value assessment and reimbursement. Figure 5 covers a range of countries in Europe, Asia Pacific, and the Americas and identifies wide variation in current funding of NGS. For example, in Brazil, NGS may only be covered through private insurance in specific circumstances. In comparison, in Germany there is broad reimbursement coverage of NGS panels. Looking across different countries, there is a lack of clear funding structures for NGS testing that impedes patient access.
Figure 5. NGS access challenges across key global countries
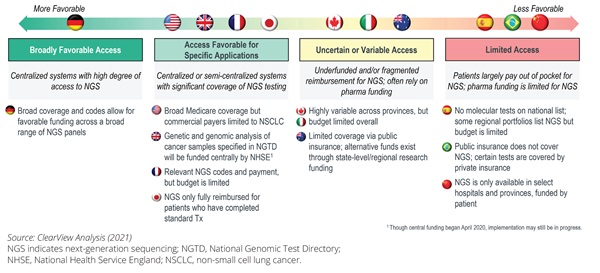
"A key barrier to next-generation sequencing access is the approach to funding, including the decision making on value assessment and reimbursement."
There are further barriers to the value assessment process for NGS. Many health systems do not provide a tailored pathway to assess the value of NGS testing. A further issue is identified if the diagnostic value assessment is included in the value assessment for the corresponding therapy. Due to the dynamic nature of NGS testing and the test outcome providing multiple theoretic treatment pathways, there is no one-to-one relationship between the NGS test and the value of one single therapy. Many HTA agencies, however, continue to include the value assessment of the diagnostic in the assessment of treatments, often resulting in an unfavorable outcome in the economic model. This approach is not appropriate to assess the value of a larger NGS panel,16 as the healthcare system benefits of NGS are broader than those that can be directly attributed to the therapy under assessment.17
• Stakeholder awareness:
The use of NGS will be dependent on the treating physicians’ awareness of how NGS could benefit their patients and direct therapy. In addition, there may be a need for clinician awareness on availability of NGS technology in their local resource setting.
Because NGS is a complex and technical subject, there is a need for education for all stakeholders to facilitate a shared understanding of the clinical benefit of NGS testing (both in cases where there is an associated treatment and where there isn’t), the barriers to access, and the impact these have on patients. Physicians need updated information and evidence on the approaches to diagnostic testing.14 One survey that interviewed European Public Health Association members found that there is a need to increase awareness of genomics among European public health professionals, as only 28.9% correctly identified all medical conditions for which there is (or is not) evidence for implementing genomic testing.18 As discussed in the ISPOR panel, there is a lack of understanding among stakeholders regarding the number of applications of NGS where treatments exist.
"A lack of awareness of the benefits that next-generation sequencing-based testing can provide is stifling the debate."
Additionally, NGS can cause concern for patients regarding how data will be shared (eg, around hereditary data impacting family members), the potential for lapses in data security around NGS testing, and how the information will be used (whether there could be implications on the premiums of their insurance, for example).14 A solid legal framework for data ownership and data usability that applies across borders will therefore be required.19 Furthermore, patients could benefit from a better understanding of the ramifications of NGS, including how this affects treatment options14 and the consequences for family members and relatives.14 Health literacy, as called out in Europe’s Beating Cancer Plan, should play a more important role not only in prevention but also in the context of precision medicine and respective NGS testing.20
Prioritizing policies to improve access to NGS testing in oncology
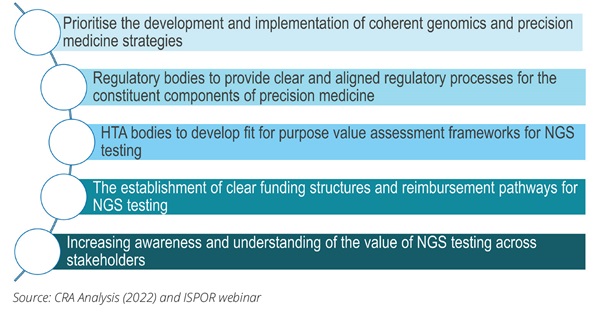
Drawing on the discussion at the ISPOR webinar and panel discussion, there are a wide range of potentially important policies that would support access to NGS.21 The ISPOR panel discussion identified 3 policy initiatives that could be prioritized.
1. Establishing an effective governance framework for NGS. This includes encouraging governments to develop a national cancer genomics strategy that should allocate sufficient funding for investment in NGS infrastructure (eg, instrumentation and reporting software) and local expertise (eg, running the lab, report interpretation, as well as post-report services around clinical decision support and genetic counseling) to facilitate the broader uptake of NGS testing, which in turn would lead to a reduction in the cost of performing an NGS test.17 Appropriate funding supporting the implementation stage of the national genomics strategy would ensure that the targets laid out in the strategy are being met and that the involved stakeholders are aware of the progress and milestones. This comes with a focus on reducing disparities in access to genomic testing within the country by addressing the barriers feeding such inequities and the potential for coordination across countries in sharing scarce resources to undertake testing and interpreting the results. Policy initiatives such as the European Commission’s Europe’s Beating Cancer Plan have a significant role to play. In this plan, the Commission has identified an ambition to improve cancer diagnoses through the “Cancer Diagnostic and Treatment for All” flagship initiative. This identified how NGS can facilitate efficient genetic profiling of tumor cells, allowing cancer centers to share cancer profiles and apply the same or similar diagnostic and therapeutic approaches to patients with comparable cancer profiles.
2. Optimizing approaches and frameworks used to assess the value of NGS and the allocation of funding.
This includes the call to payers to introduce a fair value assessment for NGS testing that acknowledges the broad benefits that emerge from such testing. This would recognize the value of NGS and acknowledge that the cost of NGS testing cannot be apportioned to one single treatment, recognizing the value to the cancer diagnostic and treatment journey as a whole. HTA agencies should design and implement adaptable frameworks that have more flexibility regarding the evidence requirements for NGS tests across the various test applications that incorporate the latest scientific advancements in terms of evidence.14,17 Alignment between the HTA process and reimbursement channel is an important consideration. Where necessary, establish a separate funding and reimbursement pathway for NGS testing that provides the required flexibility and can account for changes in the value of the NGS test across time. Aligning the funding and reimbursement pathways of NGS testing would ensure that the NGS test and the recommended treatment can be made available to patients at the same time, reducing access delays.
3. NGS will transform healthcare. Therefore, political action is needed to create a strategic framework that addresses the different aspects of funding, education, and implementational and legal considerations, and involves all stakeholders, including policy makers, clinicians, payers, and patients.
A lack of awareness of the benefits that NGS-based testing can provide is stifling the debate. Multistakeholder education on the benefits of NGS could entail collaboration with medical societies such as ESMO and ASCO to provide healthcare professionals with education on NGS and supportive comparative evidence of the available testing options to facilitate evidence-based decisions.14 These efforts may help drive implementation of NGS testing into clinical practice by creating awareness of its clinical utility. To increase patients’ perceptions of the value and awareness of NGS testing, patients could be provided with educational materials on NGS testing and clear consent forms that indicate how and with which stakeholders the NGS data would be shared.14 Finally, there is a need for the development and implementation of legal frameworks providing clarity on the data ownership and usability of genomics data that would provide more reassurance to physicians and patients of the safety of the information. Data privacy issue—including policies regarding ownership of data, usability of genomics data, and how patient privacy can be safeguarded—are worthy of further discussion and debate.
Figure 6. Conclusions: there is a need for holistic policy intervention to address the barriers hindering access to NGS testing
Key conclusions
Clinical practice is shifting towards an era of precision medicine. The use of genomic profiling of patients with technologies such as NGS has the potential to support the selection of therapies on a much larger scale than it is currently and has the potential to improve health outcomes while using healthcare resources more efficiently.
Given the nature of NGS technology and the broad barriers that it faces, there is agreement that holistic policy intervention will be needed (Figure 6), but the ISPOR panel agreed there are also clear priorities—such as establishing the governance framework for NGS and the approach to funding and reimbursement and educating all stakeholders—that require action in the shorter term.
Acknowledgment: The authors would like to thank Panos Kanavos, PhD, MSc and Oriol Solà-Morales, MSc, PhD for their contributions to the ISPOR panel.
References
- Qin D. Next-generation sequencing and its clinical application. Cancer Biol Med. 2019;16(1):4-10. doi:10.20892/j.issn.2095-3941.2018.0055
- Mosele F, Remon J, Westphalen CB, et al. Recommendations for the use of NGS for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. 2020;31(11): 491-1505. doi:https://doi.org/10.1016/j.annonc.2020.07.014
- Chakravarty D, Johnson A, Sklar J, et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO Provisional Clinical Opinion. J Clin Oncol. 2022;40(11): 1231-1258. https://ascopubs.org/doi/ full/10.1200/JCO.21.02767
- Rozenblum A, Ilouze M, Dudnik E, et al. Clinical impact of hybrid capture–based next-generation sequencing on changes in treatment decisions in lung cancer. J Thorac Oncol. 2017;12 (2): 258-268. https://doi. org/10.1016/j.jtho.2016.10.021
- Oliveri S, Ferrari F, Manfrinati A, Pravettoni G. A systematic review of the psychological implications of genetic testing: a comparative analysis among cardiovascular, neurodegenerative and cancer diseases. Front Genet. 2018;9:624. doi: 10.3389/fgene.2018.00624
- Kamps R, Brandão RD, van den Bosch BJ, et al. Next-generation sequencing in oncology: genetic diagnosis, risk prediction and cancer classification. Int J Mol Sci. 2017;18(2):308. doi: 10.3390/ijms18020308
- Hamblin A, Wordsworth S, Fermont JM, et al. Clinical applicability and cost of a 46-gene panel for genomic analysis of solid tumours: retrospective validation and prospective audit in the UK National Health Service. PLoS Med. 2017;14(2):e1002230. doi: 10.1371/journal. pmed.1002230. PMID: 28196074; PMCID: PMC5308858
- Genomics England. About Us. Accessed August 5, 2022. https://www. genomicsengland.co.uk/about-genomics-england/
- genomeDE. National Strategy for Genomic Medicine. Accessed August 5, 2022. https://www.bundesgesundheitsministerium.de/en/en/ international/european-health-policy/genomde-en.html
- European Commission. European ‘1+ Million Genomes’ Initiative. Updated May 25, 2022. Accessed August 5, 2022. https://digital-strategy.ec.europa.eu/en/policies/1-million-genomes#:~:text=The%20%EF%BF%A2%EF%BE%80%EF%BE%991%2B%20Million%20Genomes%EF%BF%A2%EF%BE%80%EF%BE%99,healthcare%20and%20health%20policy%20making
- European Commission. Europe’s Beating Cancer Plan: Communication from the Commission to the European Parliament and the Council. Accessed August 5, 2022. https://ec.europa.eu/health/sites/ default/files/non_communicable_diseases/docs/eu_cancer-plan_en.pdf
- European Federation of Pharmaceutical Industries and Associations. Unlocking the potential of precision medicine in Europe—improving cancer care through broader access to quality biomarker testing. Published February 23, 2021. Accessed August 5, 2022. https://www. efpia.eu/news-events/the-efpia-view/statements-press-releases/ unlocking-the-potential-of-precision-medicine-in-europe-improving- cancer-care-through-broader-access-to-quality-biomarker-testing/
- Gill J, Fontrier AM, Miracolo A, Kanavos P. Access to personalised oncology in Europe. Published November 2020. Accessed August 5, 2022. https://www.lse.ac.uk/business/consulting/reports/access-to- personalised-oncology-in-europe
- Faulkner E, Holtort AP, Walton S, et al. Being precise about precision medicine: what should value frameworks incorporate to address precision medicine? A report of the Personalized Precision Medicine Special Interest Group. Value Health. 2020;23(5):529-539. doi: 10.1016/j. jval.2019.11.010
- Phillips KA. Methods for moving the evaluation of precision medicine into practice and policy.Value Health. 2020;23(5):527-528. doi: 10.1016/j. jval.2020.03.002
- Burns BL, Bilkey GA, Coles EP, et al. Healthcare system priorities for successful integration of genomics: an Australian focus.Front Public Health. 2019;7:41. doi:10.3389/fpubh.2019.00041
- Rodes Sanchez M, Henderson N, Steuten L. Bridging the gap: pathways for regulatory and health technology assessment of histology independent medicines. OHE Consulting Report. 2020. https://www. ohe.org/publications/bridging-gappathways-regulatory-and-health- technology-assessment-histology-independent
- Rosso A, Pitini E, D’Andrea E, et al. Genomics knowledge and attitudes among European public health professionals: results of a cross-sectional survey.PLoS One. 2020;15(4):e0230749. https://doi.org/10.1371/journal.pone.0230749
- Kaufman D, Bollinger J, Dvoskin R, Scott J. Preferences for opt-in and opt-out enrollment and consent models in biobank research: a national survey of Veterans Administration patients. Genet Medicine. 2012;14(9):787-794. doi: 10.1038/gim.2012.45. Epub 2012 Apr 26.
- Brand A, Brand H. Health literacy and public health genomics: innovation management by citizens. Public Health Genomics. 2011;14(4- 5):193-194. https://doi.org/10.1159/000324237
- Horgan D, Curigliano G, Reiss O, et al. Identifying the steps required to effectively implement next-generation sequencing in oncology at a national level in Europe. J Pers Med. 2022;12(1):72. https://doi. org/10.3390/jpm12010072
Explore Related HEOR by Topic

