The ISPOR 2023 Patient Representatives Roundtable – Europe took place on November 12th in Copenhagen, Denmark, with 16 stakeholders from 14 European countries attending. The attendees included patient representatives, researchers and academics, industry representatives, and regulatory and government participants. The theme for this year's roundtable was 'Joint Clinical Assessment (JCA): Will it improve the patient experience of involvement in HTA?' The discussion topics included:
- an overview of JCA and PICOs (patient/population, intervention, comparison, and outcomes),
- patient involvement in HTA (Health Technology Assessment) in Europe (recommendations from a 360° perspective view of current patient involvement practices)
- JCA training for patients (European Capacity Building for Patients – EUCAPA project).
Joint Clinical Assessment (JCA): Will it improve the patient experience of involvement in HTA?
Sophie Werkö, Manager of International Relations & Patient Engagement at the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) in Stockholm, Sweden, Dr. Anke-Peggy Holtorf, Steering Committee member of the Patient and Citizen Involvement in HTA Interest Group (PCIG) at HTAi and Managing Director of Health Outcomes Strategies GmbH in Basel, Switzerland, and Valentina Stramiello, Director of Programmes of European Patients' Forum in Brussels, Belgium, gave presentations on JCAs (Joint Clinical Assessments) and whether their use will improve the patient experience of HTA involvement.
In her presentation "Overview of JCAs and PICO," Sophie Werkö discussed the elements of PICO (population, intervention, control/comparison, and outcomes), JCA, and the new EU legislation on HTA in Europe that will be implemented in January 2025. Sophie emphasized that outcomes must be patient-relevant; otherwise, they lose their function. The highlighted challenges for JCA and PICO include timelines, limited access to data, synthesis, and analysis, difficulty targeting "control/comparison" in PICO, and challenges involved with influencing at the EU and national levels. Sophie concluded that JCA will enhance the patient experience by increasing the patient's voice despite the challenges.
The next speaker, Dr. Anke Peggy Holtorf, discussed "360-degree project results and EU-JCA process from a patient involvement perspective." Anke Peggy presented a project led by Health Technology Assessment International (HTAi), which sought to identify good practices in patient involvement in HTA by seeking the perspectives, experiences, and views of HTA organizations, patient stakeholders, and industry representatives. A two-track approach consisting of detailed process perceptions and European-wide perspectives encouraged stakeholder groups to co-create recommendations based on the outputs of both tracks. The outcomes from this project highlighted that patients need to be aware of HTA involvement opportunities and how to get involved, need more knowledge (capability) or resources (capacity), and some patient organizations need to consider HTA involvement a priority. Key recommendations presented include:
- Increasing collaboration efforts between HTA and patient organizations.
- Providing more guidance and active outreach.
- Implementing early alert systems to improve preparation.
- Increasing clarity on the purpose of patient involvement.
- Building templates for patient input that are adaptable to the specific target groups.
To attain consistency and quality of patient involvement, it is instrumental to establish advisory boards with patient representatives, provide proper training and guidance to both patients and researchers, collaborate on training programs and materials, ensure effective and consistent communication, and promote transparency, accessibility, and feedback opportunities between patient stakeholders and HTA organizations.
In her presentation "JCA Training for Patients and EUCAPA," Valentina Strammiello discussed EUnetHTA provisions regarding patient experts and their associated challenges. The speaker also introduced the goals of a European Capacity Building for Patients (EUCAPA) project in addressing patient groups training needs.
The selection criteria for patient experts in JCA highlighted two key aspects - relevant expertise and experience of the disease/area of treatment and broad European knowledge of the therapeutic area. However, there are challenges in involving patient experts, such as a need for profiles on the repository of patient experts and conflict of interest (COI) criteria, which can restrict patient participation and timely involvement.
To overcome these challenges, specific enablers for patient involvement must be addressed, such as clarification of patient expert criteria and precise interpretation of COI criteria. Goals for the EUPACA project include building the skills and knowledge of patient advocates participating in HTA, preparing organizations to respond to the requests for patient experts from European HTA Cooperation, and ensuring patients and patient organizations have the necessary knowledge of the HTA process, its legal framework, and HTA methods needed to participate and advocate. Valentina presented similarities and differences between EUCAPA and HTA4Patients, coordinated by the European Patients Academy on Therapeutic Innovation (EUPATI). Some similarities include that both projects are funded by the EU4HEALTH programme, and they have the same general objectives and target groups. Some differences include the timelines, deadlines, and formats of the training.
Targeted Open Discussion
Following the speakers' presentations, the attendees participated in a targeted open discussion and interactive activity. Many attendees emphasized that the lack of sufficient training or support available for patient groups is the primary challenge and obstacle to involving patients in JCAs. Additionally, they highlighted that the most effective areas for patient input in future JCAs are PICO scoping at the national level. The open discussion provided valuable insights into enhancing patient involvement in JCAs. The discussion was completed by use of a Mentimeter survey, the results of which can be accessed below.
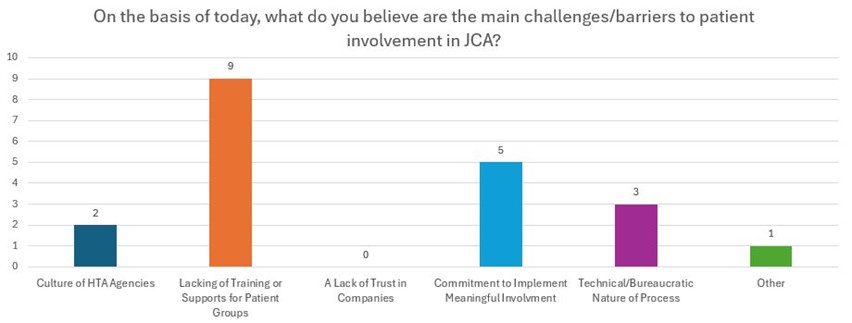
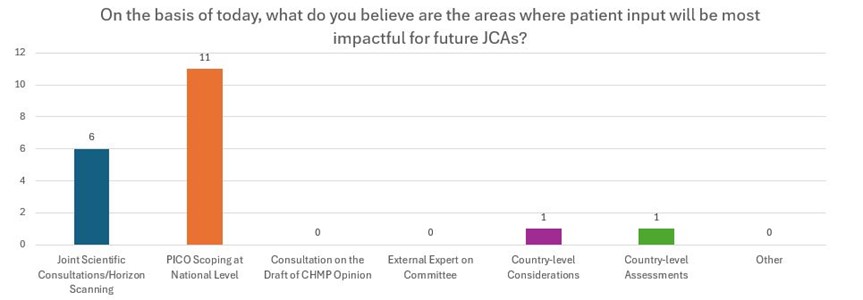
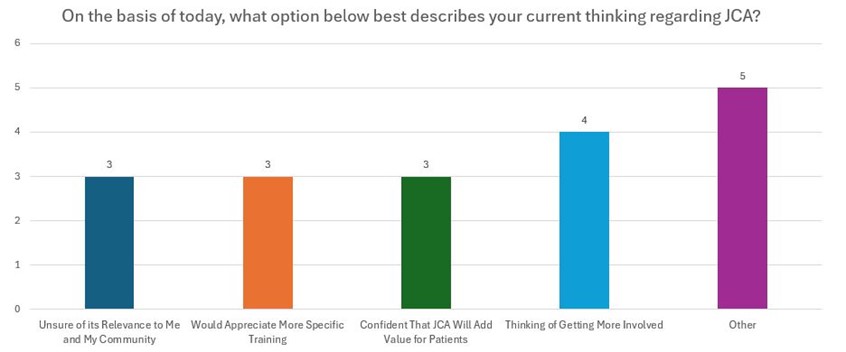
Conclusions
JCAs are a significant development in the healthcare industry as they foster collaborative evaluations to enhance the patient experience in HTA. JCAs integrate multi-disciplinary expertise and patient input to amplify patients' voices in healthcare decision-making. However, despite the benefits of involving patients in JCAs, there are challenges in incorporating patient perspectives, such as barriers to patient participation, data accessibility, data quality, resource constraints, adapting assessment methods, and regulatory compliance. Nevertheless, including patients in JCAs can lead to a more patient-centered approach to HTA, resulting in healthcare decisions that better align with patients' diverse needs and experiences.

Acknowledgments
This summary was developed by Sahar Alam, MPH, Manager, ISPOR Scientific and Health Policy Initiatives. We would like to thank the following individuals for their direction and leadership – Derick Mitchell, Ph.D., CEO of Irish Platform for Patient Organisations, Science & Industry (IPPOSI) and Chair, ISPOR Patient Representatives Roundtable – Europe, Clarissa Cooblall, MPH, Director of ISPOR Scientific and Patient Initiatives, and Laura Pizzi, PharmD, MPH, ISPOR Chief Science Officer. We greatly appreciate all speakers – Sophie Werkö, Manager of International Relations & Patient Engagement at the Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU) in Stockholm, Sweden, Dr. Anke-Peggy Holtorf, Managing Director of Health Outcomes Strategies GmbH in Basel, Switzerland, and Valentina Strammiello, Director of Programmes of European Patients' Forum in Brussels, Belgium, and all attendees for their participation and valuable contributions.
Additional Information:
The ISPOR Patient Representatives Roundtable provides a platform for patient representatives to discuss issues and challenges of patient involvement in healthcare research and decision-making processes with other key stakeholders such as researchers, HTA bodies, payers, decision-makers and policymakers, and pharmaceutical industry representatives. To learn more about ISPOR's Patient Engagement in Health Economics and Outcomes Research (HEOR) initiative, visit our website.