Wilson Follador, PhD, Pharm D, Pharmacist, Sano-Efiko Consulting Ltd and ISPOR – Brazilian Chapter Vice-President.
Stephen Stefani, MD, Oncologist, HEOR Professor, ISPOR – Brazilian Chapter President
Brazil has a large public healthcare system, called the Unified Health System (SUS), whose main characteristics are to be universal, comprehensive, and free of charge on covering for about 214 million Brazilian inhabitants. However, the average per capita spending on health care by SUS is too low, having been about US$ 278.89 (BRL 1,447.31) per capita [1] in 2020. For a simple comparison, according to data from OECD, the same per capita expenditure in United Kingdom was US$ 4,246 [2], which is paradoxical when one recognizes that the British model was one of the greatest inspirations for the creation of SUS in 1990.
The HTA (Health Technology Assessment) is clearly essential for any collective health systems in order to make cost-effective and sustainable choices for health coverage. In a scenario of low financial availability, as SUS, this tool is even more necessary. The Brazilian Ministry of Health implemented the first HTA commission in 2006, under the denomination CITEC (Commission for the Incorporation of Technologies). Despite being a good initiative, this commission was less effective due to the lack of adequate conditions to carry out the many requests it has received since its creation. The HTA process in Brazil became more structured in 2011, when CONITEC (National Commission for the Incorporation of Technologies in SUS) was created, from then on with all the legal and structural basis to carry out the evaluations.
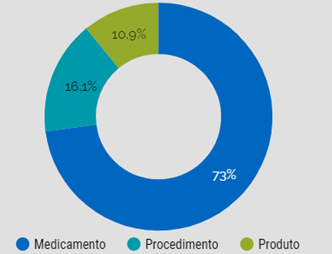
Since its beginning, CONITEC has received 1,098 requests for evaluation of medicines, health products (devices, equipment, diagnostic tests, etc.) and medical procedures, with 776 decisions completed3. The distribution of the analyses by each type of technology is described in Figure 1.
Figure 1: Percentage of assessments grouped by the main nature of technology [3].
From the total evaluations carried out, CONITEC has approved 407 request (62%) and rejected 249 (38%). It should be mentioned that among the 249 rejections, 149 were refused before the technical analysis due to the non-compliance of documentation requirements predefined by CONITEC. It is worth mentioning that 55% of the requests sent to CONITEC were originated internally from the Brazilian government (mainly the Ministry of Health, State Health Secretariats and agents of the Brazilian judicial system), while 45% came from external agents (mainly technology industries, but also medical societies, patient organizations, etc.).
Until the first half of 2022, all the facts reported occurred with CONITEC formatted by an executive secretariat, a body of technicians supported by REBRATS (Brazilian Network for Health Technology Assessment) and a committee, called "Plenary", in charge of taking the final judgment decisions regarding each claim.
It was from the second half of 2022 that two significant changes in CONITEC's processes began. The first of all was the publication, in 2022, of Law No 14.313 and Decree No 11.161, which changed the CONITEC’s structure. Instead of a single team in charge of evaluating all HTA requests, 3 committees were created, divided by large areas of health technologies: "Medicines", "Products and Procedures", and "Clinical Protocols and Therapeutic Guidelines". These committees assumed the functions of the former "Plenary" in making decisions regarding the approval or not of HTA claims. Each committee consists of 15 members, most of whom are linked to the main secretariats of the Ministry of Health, added by representatives of State Secretariats and Municipal Health Secretariats, ANVISA (National Health Surveillance Agency), ANS (National Agency for Supplementary Health), the Federal Council of Medicine, the Brazilian Medical Association and NATS (Center for Health Technology Assessment).
Another important change that came into force from this Law is that the claims approved by CONITEC became mandatorily approved for inclusion in the ANS (National Agency for Supplementary Health, a governmental agency that is responsible for the regulation of private health insurance). However, it is important to know that ANS does not impose coverage of any product (drugs, equipment, devices and the like), but only medical procedures. Thus, even if CONITEC approves a specific drug or medical device, ANS will not be required to manifest itself, unless this technology requires a specific medical procedure. To make this clearer, we can cite as an example the technologies that refer to minimally invasive surgeries, which may require the creation of a specific item in the ANS list that differentiates the minimally invasive method from the open surgery method.
Finally, we should mention that for about two years now, due to legal obligations, ANS has been making exceptions to the rule of only imposing coverage for procedures, through the imposition to private health insurers of coverage for some medicines (e.g., anticancer drugs for oral use and a few other medicines), but in lists that are still quite restricted and under strict protocols for use.
The last item to be mentioned about recent changes in HTA in Brazil refers to the implementation of an ICER threshold. There was no reference for the ICER threshold in Brazil the past because it was understood that it would be hard defining a value that was fair and adequate to guide HTA judgements in SUS. However, the Law number 14,313 established that HTA in Brazil should define such thresholds. So, after studies, CONITEC has defined that the ICER threshold in Brazil would be 1 GDP per capita for health technologies in general and up to 3 GDP per capita for technologies to be used in rare diseases. This represents a total of BRL 46,154.60 (about US$ 9,141.52, using the exchange rate of US$1 = BRL 5.0489 on 4/20/2023) for most of health technologies and BRL 138,463.80 (about US$ 27,424.56) for rare diseases, considering the GDP per capita as noticed by IBGE – Brazilian Institute of Geography and Statistics [4]. And it is important to mention that the GDP per capita applied in this text was obtained from IBGE, but it is not sure if CONITEC will use the same value or some other reference.
Observing the reality of several countries that have already adopted the reference of 1 GDP per capita as the ICER threshold to define a cost-effective technology, the value defined by CONITEC does not escape the standards, but it is believed that, under the point of view of manufacturers and some other parts of society, this value will be too low to be used as a standard in the evaluation of technologies coming from developed countries, whose present GDP per capita value is significantly higher than the Brazilian one and whose reimbursement values are commonly much higher than those practiced by the Brazilian health system, in addition to considering indirect costs.
It is still too early to express opinions about how these changes will impact HTA in Brazil, but there is reasonable concern about the implementation of these measures in a SUS funding scenario that is still visibly underestimated.
References:
- Brasil - Secretaria do Tesouro Nacional - Ministério da Fazenda. Relatório Resumido da Execução Orçamentária (RREO) - União. Published 2023. Accessed April 20, 2023. https://www.tesourotransparente.gov.br/temas/contabilidade-e-custos/relatorio-resumido-da-execucao-orcamentaria-rreo-uniao
- OECD - The Organisation for Economic Co-operation and Development. Health at a glance 2021. Published 2021. Accessed April 20, 2023. https://www.oecd.org/health/health-at-a-glance/
- Zimmerman I, CONITEC - Secretaria Executiva. CONITEC em números. Published 2023. Accessed April 20, 2023. https://lookerstudio.google.com/embed/reporting/afb9eff6-9786-4172-a4f0-a403580ff5f6/page/PzCbB
- Amorim D, Nader V. IBGE: PIB per capita alcançou R$ 46.154,6 em 2022. Estadão e-Investidor. Published 2023. Accessed April 20, 2023. https://einvestidor.estadao.com.br/ultimas/pib-per-capita-2022-ibge