Is Europe Ready to Value and Reimburse the Next Medical Frontier?
Aakash Bipin Gandhi, BPharm, Department of Pharmaceutical Health Services Research, University of Maryland, Baltimore, MD, USA; and Vasco Pontinha, MPharm, MA, Department of Pharmacotherapy and Health Outcomes, Virginia Commonwealth University School of Pharmacy, Richmond, VA, USA
The session focused on discussing challenges surrounding health technology assessments (HTAs) and reimbursement for gene therapies in Europe. This session had clear objectives, which included reviewing the fundamentals of gene therapy and its applications in treating rare diseases, discussing financial challenges posed by these expensive treatments, examining novel reimbursement strategies for gene therapies that are currently being used around the world, and discussing potential approaches to overcome challenges associated with value-based assessment of gene therapies.
“The promise of gene therapy is very clear: A potential to cure very debilitating and burdensome diseases." —Omar Dabbous, MD, MPH
Upfront Costs and Long-Term Benefits
Briefly, gene therapy encompasses 4 approaches, such as transfer (replacing a dysfunctional or missing gene), addition (targeting a specific disease to supplement target therapy), inhibition (silencing expression of a mutant gene), or editing (a patient’s genome to correct a mutant gene). Omar Dabbous, MD, MPH, Global HEOR and RWE, Novartis Gene Therapies, Bannockburn, IL, USA, talked about rapid advances that have been made with gene therapies, especially the spike in clinical trials and Investigational new drug applications since 2018. According to Dabbous, “The promise of gene therapy is very clear: A potential to cure very debilitating and burdensome diseases. However, there are concerns associated with its long-term benefit and high costs.”
Additionally, it appears that the focus of HTA organizations and society at large lies heavily on the upfront costs rather than on the long-term benefits. Therefore, Dabbous argued that, “The methods that have traditionally been used to assess the value of medications to treat chronic conditions need to be adapted to the context of gene therapy. The assessment of the clinical effectiveness, underlying population characteristics, discounting, valuation of health outcomes, time horizon, and extrapolation of effects beyond clinicals trials require a tailored approach.” For example, while the cost-per-QALY provides a starting value, it may fail to showcase any long-term value yielded by gene therapy.
“Given that gene therapy targets diseases requiring constant care, shouldn’t the value of such treatments also encompass the impact on patient caregivers?” —Michael Drummond, MCom, DPhil
Assessing the Value of Gene Therapies
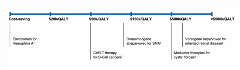
Michael Drummond, MCom, DPhil, Centre for Health Economics, University of York, York, United Kingdom, discussed both current and proposed methods for assessing the value of gene therapies. Based on the assumption that “drug prices should reflect value, there may be elements of value that are currently missing in the economic evaluation of gene therapies.” For example, it is possible that the cost-per-QALY does not fully reflect the value provided by gene therapies. Researchers may also require information on items such as the value of knowing, fear of contagion, insurance value, severity of disease, value of hope, real option value, equity, and scientific spillovers to comprehensively determine the value for gene therapies. Drummond made the case for including these elements in the economic evaluation of gene therapies, or alternatively increase the cost-per-QALY threshold. Additionally, he encouraged discussions around the inclusion of such elements to measure value at a societal level. There is a high-level of uncertainty surrounding the long-term effects of gene therapies as these are usually used for rare conditions in pediatric populations and rely on evidence generated from single arm trials. Drummond also argued that, “Given that gene therapy targets diseases requiring constant care, shouldn’t the value of such treatments also encompass the impact on patient caregivers?” It appears that there are no specific value assessment guidelines for gene therapy. Some HTA agencies seem to be able to adapt value assessment guidelines for highly specialized technological products. NICE, for example, may consider higher cost-per-QALY thresholds, up to £300,000 to evaluate gene therapies. To a certain extent, Drummond questioned the pertinence of giving special treatment status to gene therapies. An ICER report demonstrated how some of the therapies are even cost-saving (eg, hemophilia A) and others, typically uber-expensive like those for spinal muscular atrophy, actually yield cost-per-QALYs close to high end of the current thresholds (Figure 1).
Figure 1. Overview of Selected ICER Reviews for Gene Therapies
“Different payment methods have different trade-offs. Ultimately, increased cooperation and dialogue between stakeholders can aid in the implementation of a model best suited for a specific target population.” —Johann-Matthias Graf von der Schulenburg, PhD
Considerations for Affordability and Reimbursement
Johann-Matthias Graf von der Schulenburg, PhD, Leibniz University Hannover, Hannover, Germany stressed the need to “think about how we can afford expensive treatments such as gene therapies and how they should be reimbursed.” These questions are further complicated due to the uncertainty associated with gene therapies such as their long-term efficacy, their ability to improve life expectancy for patients, and difficulties in choosing a reference group against which we can evaluate their cost-effectiveness. To meet challenges surrounding reimbursements for these treatments, several innovative payment models such as performance-based risk sharing, credit systems, consumer mortgage, patient assistance subsidies, and health coins can be adopted. Based on a comparison of features involved in each of these strategies, the pay-for-performance model seems to be the most financially attractive and feasible to implement. As a key take-home message, Schulenburg stated that “Different payment methods have different trade-offs. Ultimately, increased cooperation and dialogue between stakeholders can aid in the implementation of a model best suited for a specific target population.”
"The level of evidence considered for approval and reimbursement of gene therapies today will not probably be accepted in the future.” —Mondher Toumi, MD, MSc, PhD
Given the potential for significant long-term benefits, access to gene therapies is an urgent and critical issue for patients with rare diseases. Mondher Toumi, MD, MSc, PhD, Public Health Department, Aix-Marseille University, Paris, France, discussed how pharmaceutical companies should consider the key decision-making drivers in the development phase to help with an efficient regulatory review process for final approval. These include thinking of questions such as, “Are there alternative treatment options? How big is the benefit effect size? How long does the benefit last?” The quality of clinical evidence prior to final approval and ability to generate post-approval evidence seem critical for a HTA decision. Toumi also discussed the main criticisms from HTA bodies when assessing gene therapies. Most refer to study design (eg, single arm), short-term duration of the clinical trials, and incorrect choice of historical comparators. Thus, Toumi cautioned, pharmaceutical companies should be aware that the “level of evidence considered for approval and reimbursement of gene therapies today will not probably be accepted in the future.”
Conclusion
To conclude the session, Dabbous shared some key take-home messages for the audience. First, there is a need for a quick adoption of innovative payment models to ensure efficient reimbursement for gene therapies. Second, healthcare systems should be incentivized for investing in gene therapies that offer potential long-term benefits for patients. Third, there is a need for the use of real-world evidence to better support health technology assessments for gene therapies. Fourth, there is a need for increased efforts from governments to ensure early dialogue between gene therapy manufacturers and payers that can help streamline access to these novel medications for the public.
Figure 2. Panelists (clockwise): Omar Dabbous , Johann-Matthias von der Schulenburg Mondher Toumi, and Michael Drummond.
Explore Related HEOR by Topic