From Concept to Commercialization: AI’s Emerging Role in HEOR
 Grace E. Fox, PhD, OPEN Health, New York, NY, USA; Shea O’Connell, PhD; Craig Bennison, MSc, OPEN Health, London, England, UK; Siddharth Kakked, MBBS, MPH; Kelly McCarrier, PhD, OPEN Health, New York, NY, USA; Elisabeth Fenwick, PhD, OPEN Health, London, England, UK
Grace E. Fox, PhD, OPEN Health, New York, NY, USA; Shea O’Connell, PhD; Craig Bennison, MSc, OPEN Health, London, England, UK; Siddharth Kakked, MBBS, MPH; Kelly McCarrier, PhD, OPEN Health, New York, NY, USA; Elisabeth Fenwick, PhD, OPEN Health, London, England, UK

Although still nascent in health economics and outcomes research (HEOR), artificial intelligence (AI) has the potential to reshape the field. Already, AI applications such as machine learning and statistical learning, along with automation tools like rules-based algorithms, are transforming how data are synthesized, interpreted, and communicated.1-3 There has been some uptake in HEOR, but it is uneven, constrained by technical immaturity, limited validation, and uncertainty as to regulatory expectations.4,5 Compounding these issues, many HEOR stakeholders lack the expertise to critically evaluate AI-generated evidence.
Stakeholders across HEOR would benefit from clarity on where AI-based technologies are approaching routine use and where their use remains experimental. In addition, understanding how AI applications can be integrated into the product life cycle can help organizations prioritize investment, plan operations, and ensure alignment with emerging standards.
With input from subject matter experts across our organization, we assessed the state of AI deployment across HEOR domains (eg, evidence synthesis, economic modeling, real-world data [RWD], market access, patient-centered outcomes). In describing how AI and automation have been deployed in HEOR activities, we drew upon peer-reviewed and gray literature, internal case studies, and relevant regulatory and health technology assessment (HTA) guidance.
The subject matter experts classified AI use by activity (eg, literature review) within their specific HEOR domain according to life cycle phase of use (ie, early phase, prelaunch, launch, and postlaunch). Each use of AI was rated in terms of its (1) technical maturity (validation and scalability); (2) regulatory and HTA acceptance (extent of recognition or endorsement by authorities); (3) operational feasibility (ease of integration into existing HEOR workflows); and (4) evidence of impact (measurable gains in efficiency, quality, or decision making). We rated the readiness of AI to support specific activities, using a 3-point scale: high (widely validated, feasible for immediate adoption); moderate (emerging use, requires further validation); and low (early stage or conceptual).
Here we present a summary of our findings divided by phase (see Figure).
Figure. Expert-led mapping
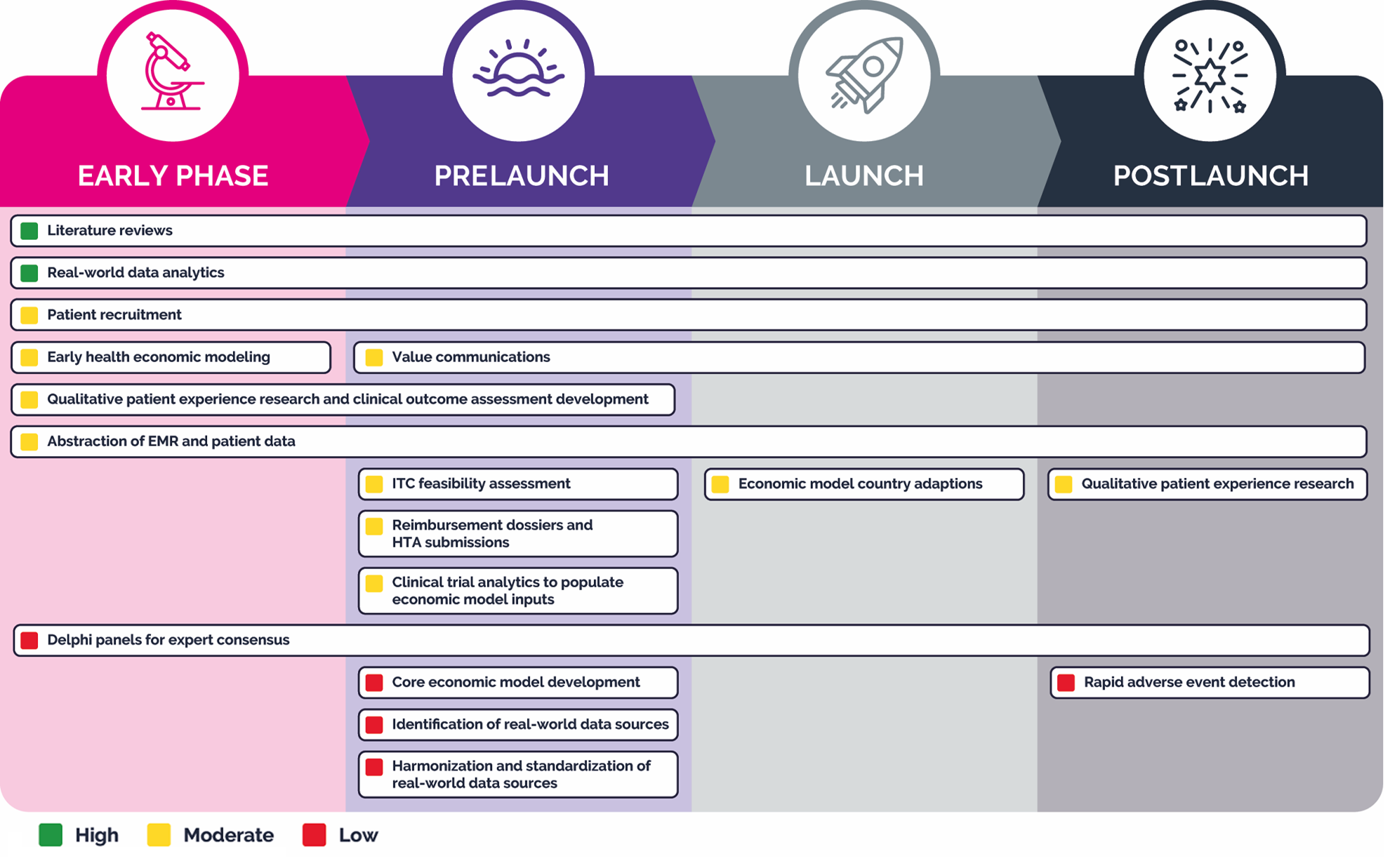
EMR indicates electronic medical record; HTA, health technology assessment; ITC, indirect treatment comparison.
Early Phase
For structured, repeatable early phase activities such as literature reviews4,6-11 and RWD analytics using free-text data,12-14 AI applications have demonstrated a high state of readiness. In literature reviews specifically, AI can support the process from protocol development through reporting, with scope and extent of use varying by review type.4,6-11
AI is also being considered for use in qualitative patient experience research and clinical outcome assessment development15,16 to support transcript coding of qualitative patient experience data, including concept elicitation, cognitive interviews, and patient journey studies, but shows only moderate readiness.
Understanding how AI applications can be integrated into the product life cycle can help organizations prioritize investment, plan operations, and ensure alignment with emerging standards.
Another early phase activity where AI shows moderate readiness is the conceptualization and generation of simple economic models to support pricing and positioning.4,17 AI also shows moderate readiness for extracting structured data from clinician notes, case reports, lab reports, and other unstructured clinical text for the abstraction of electronic medical record and patient data.18,19
AI may aid in patient recruitment for noninterventional trials,18,20 as it can access multiple healthcare data sources to scan digitized patient records, chatbots, and web platforms to assess individuals’ eligibility to participate in large, real-world studies.18,20
AI cannot yet be employed reliably to summarize and analyze panelist input between Delphi rounds21,22 or to participate alongside human experts in drafting consensus statements. Currently, these applications of AI have low readiness, with human oversight and consensus validation needed.21,22
Prelaunch
In the prelaunch phase, AI can support literature reviews with high readiness.4,6-11 It also shows high readiness for RWD analytics, where generative models can be used for extracting free text, generating structured queries, and drafting statistical coding.12-14
AI-assisted development of reimbursement dossiers and HTA submissions represents a major efficiency frontier, with moderate readiness.4,23-26 AI can be leveraged to develop, curate, update, and adapt reimbursement dossiers and HTA submission materials from a central source, dynamically tailoring them to the requirements of different markets and agencies.4,23-26 Similarly, AI-driven value communication tools can be used to develop, curate, update, and adapt relevant content and transform static single-market deliverables into dynamic, multimarket tools tailored in real time to stakeholder needs.4,23-26 This application currently demonstrates a moderate level of readiness.
Potential is evident in the use of AI-augmented transcript coding of qualitative patient experience data and AI-assisted clinical outcomes assessment development.15,16 These capabilities are moderate in readiness. Using AI for feasibility assessments for indirect treatment comparisons, clinical trial analytics to populate economic model inputs,27,28 and the abstraction of electronic medical record and patient data18,19 has promise but also shows only moderate readiness at this time.
In the prelaunch phase, AI has yet to demonstrate much utility in the identification, harmonization, and standardization of RWD sources,12 in the support of Delphi panels for expert consensus, or in core economic model development,21,22 although the potential is there in all these areas.
Launch
At launch, AI can continue to support repetitive, data-heavy tasks, such as literature reviews.4,6-11 In addition, AI models can continue to support development, curation, updating, and adapting value communication materials as they do in the prelaunch phase.4,23-26 The automated adaptation of economic models29,30 to local markets also shows moderate readiness. As noted above, AI is not yet developed to a point where it can support Delphi panels for expert consensus.21,22
Postlaunch
In the postlaunch phase, AI and automation are key to managing the continuous flow of RWD and post-marketing evidence. Because many analytic workflows are standardized, generative coding models12-14 show high readiness to execute statistical analyses from text inputs and to interpret and explain study results.12,20,21 AI for literature reviews4,6-11 continues to show high readiness in the postlaunch phase.4,6-11 While there is some evidence that AI can be used for patient recruitment18,20 in real-world, postmarketing studies to cast a wide net across multiple healthcare data sources, the use case shows just moderate readiness.18,20 Similarly, AI-assisted qualitative patient research,15,16 abstraction of electronic medical record and patient data,18,19 and value communications4,23-26 continue to evolve in parallel, maintaining moderate readiness.6-10,18,19
Our read is that AI readiness mirrors the methodological structure of HEOR activities, thriving in systematic, protocol-based processes such as evidence synthesis and analytics but lagging where expert deliberation, uncertainty, and qualitative interpretation predominate.
Applications in adverse event detection31 are developing, but their utility is currently limited to academic proofs-of-concept and methodologies that require additional validation to ensure that adverse events are not missed. Likewise, AI applications show low readiness for supporting with Delphi panels for expert consensus.21
Ready or Not (Yet)
The readiness of AI to support HEOR activities is highest in domains that are structured and rules based, such as literature reviews, model updates, and routine, repeatable RWD analytics. In contrast, the readiness of AI remains low in areas where contextual judgment and consensus building, such as Delphi processes or adverse event interpretation, are required. Our read is that AI readiness mirrors the methodological structure of HEOR activities, thriving in systematic, protocol-based processes such as evidence synthesis and analytics but lagging where expert deliberation, uncertainty, and qualitative interpretation predominate.
Although several barriers hinder widespread AI adoption in HEOR—among them data privacy, intellectual property considerations, evolving regulatory and HTA guidance, and limited workforce readiness—there remain clear opportunities for advancement. Many HEOR professionals have yet to fully understand how these tools can be effectively leveraged, highlighting the need for broader education and upskilling, particularly around generative AI. Embedding AI and automation into standard HEOR workflows and leveraging hybrid human-AI models to balance efficiency benefits with methodological rigor offer pathways toward responsible adoption.
Looking ahead, the development of formal guidance from regulatory authorities and HTA agencies, along with cross-industry collaborations that promote testing, integration, and shared learning, could further accelerate adoption and standardization. It is also important to recognize that pharmaceutical research is inherently conservative, as it must be to protect patient safety. This means that adoption of AI in safety-facing or adjacent roles must be approached with particular caution, ensuring that systems are validated and reliable from the outset rather than following a typical technology sector “release and refine” model.
Our analysis highlights clear areas of maturity, which include literature reviews, RWD analytics, and model updates, while identifying the key barriers of regulatory acceptance and data governance concerns. With our research, we aim to help stakeholders prioritize investment and implement strategies that align with emerging regulatory and HTA expectations.
Acknowledgments
The authors thank Christina DuVernay of OPEN Health for editorial support in developing this article.
References
- Macia G, Ray J. Artificial intelligence in HEOR. Value & Outcomes Spotlight. 2024;10(2):33-35. https://www.ispor.org/publications/journals/value-outcomes-spotlight/vos-archives/issue/view/defining-digital-health---getting-clarity-for-heor/artificial-intelligence-in-heor
- Fleurence RL, Wang X, Bian J, et al. A taxonomy of generative artificial intelligence in health economics and outcomes research: an ISPOR Working Group report. Value Health. 2025;28(11):1601-1610. doi:10.1016/j.jval.2025.04.2167
- ISPOR—The Professional Society for Health Economics and Outcomes Research. ISPOR 2024-2025 Top 10 HEOR Trends Report. 2025. https://www.ispor.org/heor-resources/top-10-heor-trends
- Fleurence RL, Bian J, Wang X, et al. Generative artificial intelligence for health technology assessment: opportunities, challenges, and policy considerations: an ISPOR Working Group report. Value Health. 2025;28(2):175-183. doi:10.1016/j.jval.2024.10.3846
- Tachkov K, Zemplenyi A, Kamusheva M, et al. Barriers to use artificial intelligence methodologies in health technology assessment in Central and East European countries. Front Public Health. 2022;10:921226. doi:10.3389/fpubh.2022.921226
- National Institute for Health and Care Excellence (NICE). Use of AI in evidence generation: NICE position statement, version 1. Published August 15, 2024. Accessed October 10, 2025. https://www.nice.org.uk/position-statements/use-of-ai-in-evidence-generation-nice-position-statement
- Canada’s Drug Agency. Canada’s Drug Agency position statement on the use of artificial intelligence in the generation and reporting of evidence. Published April 2025. Accessed October 10, 2025. https://www.cda-amc.ca/sites/default/files/MG%20Methods/Position_Statement_AI_Renumbered.pdf
- Institute for Quality and Efficiency in Health Care (IQWiG). General Methods Version 7.0. 2023. Accessed October 10, 2025. https://www.iqwig.de/en/about-us/methods/methods-paper/
- Fox GE, Ames JT, Santpurkar N, Avissar J, Arcà E. Smarter SLRs or risky shortcuts? Perspectives from global HTA stakeholders. OPEN Health Group; 2025. https://www.openhealthgroup.com/wp-content/uploads/AI-Perceptions-Study.pdf
- Cochrane. Cochrane’s focus is on responsible use of AI in systematic reviews. Accessed October 10, 2025. https://training.cochrane.org/sites/training.cochrane.org/files/public/uploads/Ella%20Flemyng_part%203.pdf
- Thomas J, Flemyng E, Noel-Storr A, et al. Responsible AI in Evidence Synthesis (RAISE): Guidance and Recommendations. 2025. https://osf.io/fwaud/files/cn7x4
- Adams MCB, Perkins ML, Hudson C, et al. Breaking digital health barriers through a large language model-based tool for automated observational medical outcomes partnership mapping: development and validation study. J Med Internet Res. 2025;27:e69004. doi:10.2196/69004
- Huang J, Yang DM, Rong R, et al. A critical assessment of using ChatGPT for extracting structured data from clinical notes. NPJ Digit Med. 2024;7(1):106. doi:10.1038/s41746-024-01079-8
- Pan Y, Wang C, Hu B, et al. A BERT-based generation model to transform medical texts to SQL queries for electronic medical records: model development and validation. JMIR Med Inform. 2021;9(12):e32698. doi:10.2196/32698
- Food and Drug Administration (FDA). Considerations for the Use of Artificial Intelligence to Support Regulatory Decision-Making for Drug and Biological Products: Guidance for Industry and Other Interested Parties - Draft Guidance. January 2025. https://www.fda.gov/media/184830/download
- Morgan DL. Exploring the use of artificial intelligence for qualitative data analysis: the case of ChatGPT. Int J Qual Methods. 2023;22:16094069231211248. doi:10.1177/16094069231211248
- Reason T, Rawlinson W, Langham J, Gimblett A, Malcolm B, Klijn S. Artificial intelligence to automate health economic modelling: a case study to evaluate the potential application of large language models. Pharmacoecon Open. 2024;8(2):191-203. doi:10.1007/s41669-024-00477-8
- Astorino T. AI-powered non-interventional research delivers what DCTs promised. Appl Clin Trials. Published August 8, 2025. Accessed October 10, 2025. https://www.appliedclinicaltrialsonline.com/view/ai-powered-non-interventional-research-dcts
- Schneider ME. TRIALSCOPE: using AI to scale real-world data. Regul Focus. September 22, 2025. Accessed October 10, 2025. https://www.raps.org/news-and-articles/news-articles/2025/9/trialscope-using-ai-to-scale-real-world-data
- Lu X, Yang C, Liang L, Hu G, Zhong Z, Jiang Z. Artificial intelligence for optimizing recruitment and retention in clinical trials: a scoping review. J Am Med Inform Assoc. 2024;31(11):2749-2759. doi:10.1093/jamia/ocae243
- Speed C, Metwally AA. The human-AI hybrid Delphi model: a structured framework for context-rich, expert consensus in complex domains. arxiv. Published August 12, 2025. Accessed October 10, 2025. doi:10.48550/arXiv.2508.09349 https://arxiv.org/abs/2508.09349
- Calleo Y, Pilla F. Real-time AI Delphi: a novel method for decision-making and foresight contexts. Futures. 2025;174:103703. doi:10.1016/j.futures.2025.103703
- Walters J, Guerra I, Rtveladze K, et al. SA62 Evaluating generative artificial intelligence (GenAI) in health technology assessment (HTA) content generation: a proof-of-concept using Canadian Agency for Drugs and Technologies in Health (CADTH) reimbursement dossier forms. Value Health. 2024;27(12):S626. doi:10.1016/j.jval.2024.10.3143
- Jost J, Walzer S, Vollmer L. HTA113 Comparative analysis of traditional vs AI-assisted health technology assessment dossier writing. Value Health. 2024;27(12):S375. doi:10.1016/j.jval.2024.10.1938
- Dietrich ES. Artificial intelligence in key pricing, reimbursement, and market access (PRMA) processes: better, faster, cheaper—can you really pick two? J Med Econ. 2025;28(1):586-595. doi:10.1080/13696998.2025.2488154
- Reason T, Klijn S, Rawlinson W, et al. Using generative artificial intelligence in health economics and outcomes research: a primer on techniques and breakthroughs. Pharmacoecon Open. 2025;9(4):501-517. doi:10.1007/s41669-025-00580-4
- Wu Y, Klijn S, Teitsson S, Malcolm B, Jones C, Rawlinson W. Innovations in automated survival curve selection and reporting of survival analyses through generative AI. Presented at: ISPOR EU; November 17-20, 2024; Barcelona, Spain. https://www.ispor.org/docs/default-source/euro2024/cjonessurvivalanalysiss155p24143577-pdf.pdf
- Chhatwal J, Samur S, Yildirim IF, Bayraktar E, Ermis T, Ayer T. Fully replicating published Markov health economic models using generative AI. 2024. Accessed October 10, 2025. https://www.valueanalyticslabs.com/wp-content/uploads/2024/12/Fully-Replicating-Published-Markov-Health-Economic-Models-Using-Generative-AI.pdf
- Rawlinson W, Teitsson S, Reason T, Malcolm B, Gimblett A, Klijn S. Automating economic modeling: the potential of generative AI for updating Excel-based cost-effectiveness models. Presented at: ISPOR; May 5-8, 2024; Atlanta, GA, USA. https://www.ispor.org/docs/default-source/intl2024/ai-adaptations-excel-ispor-us-2024138676-pdf.pdf?sfvrsn=f85aa30_0
- Rawlinson W, Teitsson S, Reason T, Malcolm B, Gimblett A, Klijn S. Assessing the generalizability of automating adaptation of Excel-based cost-effectiveness models using generative AI. Value Health. 2024;27(12)S2. https://www.ispor.org/heor-resources/presentations-database/presentation/euro2024-4014/144576
- Algarvio RC, Conceicao J, Rodrigues PP, Ribeiro I, Ferreira-da-Silva R. Artificial intelligence in pharmacovigilance: a narrative review and practical experience with an expert-defined Bayesian network tool. Int J Clin Pharm. 2025;47(4):932-944. doi:10.1007/s11096-025-01975-3

