Unlocking Real-World Evidence for Market Access of Medical Devices and Diagnostics in Europe
Bjoern Schwander, PhD, AHEAD GmbH, Bietigheim-Bissingen, Germany; Axel Mühlbacher, PhD, Hochschule Neubrandenburg, Neubrandenburg, Germany; Stephen Duffield, PhD, MD, NICE, Manchester, United Kingdom; Sandrine Bourguignon, PhD, RWEality, Paris, France; James Lavin, Intuitive Sàrl, Aubonne, Switzerland

Introduction
The growing demand for innovative medical devices and diagnostics (MD&D) presents both opportunities and challenges for market access in Europe. While clinical trials remain the gold standard for demonstrating safety and efficacy, the need for robust real-world evidence (RWE) is increasingly recognized.
Across Europe, RWE has become an essential component in assessing the value, safety, and effectiveness of MD&D. The following examples from Germany, the United Kingdom, and France illustrate how health authorities leverage diverse RWE sources to support regulatory decisions, reimbursement, and ongoing evaluation in routine clinical practice:
Germany: A digital therapeutic (DTx) developed for type 2 diabetes management aims to enhance standard care through features like real-time glucose monitoring, personalized feedback, and lifestyle coaching.1 Although clinical trials demonstrated significant improvements in glycemic control, such as reduced HbA1c levels, payers and health technology assessment (HTA) bodies raised concerns regarding its long-term effectiveness, cost-effectiveness, and real-world patient adherence. To address these critical questions, the manufacturer implemented a comprehensive real-world evidence strategy, integrating data from multiple sources, including electronic health records that provided longitudinal patient data, wearable device data for continuous glucose monitoring, and patient-reported outcomes that captured behavioral changes and quality-of-life improvements. This systematic approach not only strengthened the evidence base but also facilitated ongoing evaluation of the therapeutic’s impact on patient adherence and healthcare utilization.
United Kingdom: A new medical device used for diagnosing complex cardiovascular conditions is entering the early value assessment (EVA) stage with the National Institute for Health and Care Excellence (NICE).2 Given its innovative design, limited trial data are available, so NICE recommends the collection of RWE through pragmatic studies and registry data to support the decision process. The manufacturer collaborates with National Health Service (NHS) providers to gather observational data on device performance, durability, and patient outcomes in routine clinical practice over several years. These real-world data help fill evidence gaps on long-term safety, effectiveness, and health economic impact, facilitating ongoing assessments as the device transitions from early access to wider NHS adoption. By systematically integrating these data sources, NICE aims to ensure that reimbursement and clinical use decisions are evidence-based and reflective of real-world performance.
Across Europe, real-world evidence has become an essential component in assessing the value, safety, and effectiveness of medical devices and diagnostics.
France: The French National Health Data System (SNDS) has been extensively used to support the postmarket surveillance and long-term assessment of implantable cardiac devices, such as pacemakers and implantable defibrillators.3 These studies leverage SNDS data to analyze device longevity, complication rates, re-interventions, and overall safety in real-world clinical practice. For example, analysis of SNDS data has demonstrated sustained safety profiles and durability of these devices over several years, providing critical evidence that informs regulatory and reimbursement decisions. This comprehensive use of real-world data ensures continuous monitoring of device performance, helping to maintain safety standards and guiding the clinical management of patients with these implants. Such examples illustrate the value of RWE in France for the life-cycle management of high-risk medical devices, supporting their safe and effective use in routine healthcare settings.
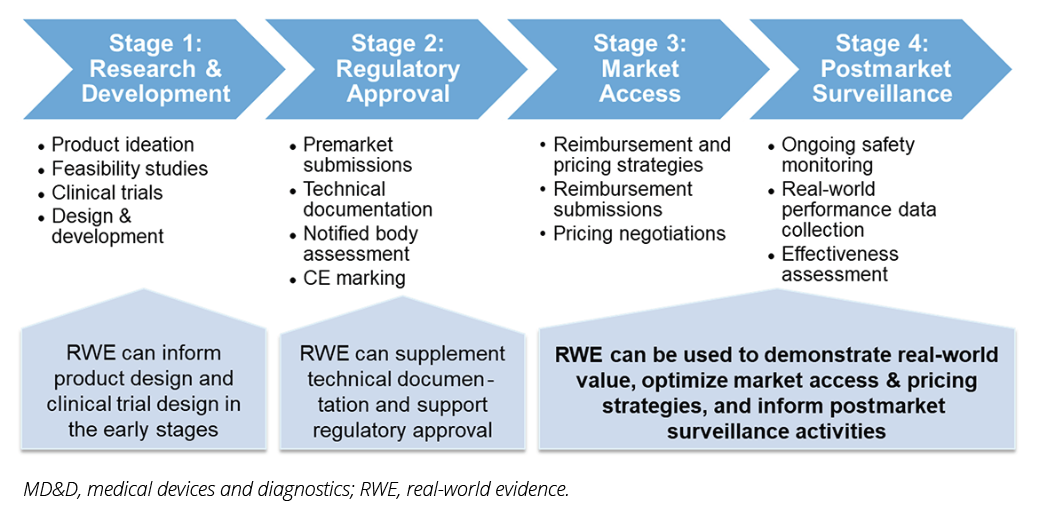
This article explores the evolving role of RWE in supporting market access for MD&Ds in Europe, drawing on insights from a recent webinar hosted by the ISPOR Medical Devices and Diagnostics Special Interest Group. As presented in Figure 1, RWE plays a significant role throughout the entire product life-cycle of MD&Ds, informing decisions and improving outcomes at each stage. The article places special emphasis on the use of RWE for market access purposes, recognizing its potential to bridge evidence gaps and foster better decision making.
Figure 1. MD&D Product Life-cycle and RWE Integration
Germany: Navigating the Strict Regulatory Landscape
Germany’s healthcare system, which is strictly regulated by the laws in the Social Code Book 5 (Sozialgesetzbuch V, SGBV), emphasizes evidence-based decision making, particularly relying on clinical trials for demonstrating causality.4
Furthermore, it was emphasized that RWE can complement clinical trial evidence, required by SGBV:
- Leveraging existing registries and data sources can help identify unmet medical needs and validate the clinical relevance of new technologies. While registries provide structured data on diagnosed conditions and treatment patterns, RWE encompasses a wider array of data sources, such as electronic health records, claims databases, and patient-reported outcomes. This integration of diverse data types helps identify unmet medical needs and validate the clinical relevance of new technologies.
- RWE can provide insights into the real-world safety and performance of medical devices, complementing data from controlled environments. This can lead to more accurate risk classifications and better-informed decisions regarding product development and approval.
Despite these opportunities, the challenges related to data availability, access, and the validation of causality in RWE studies remain critical considerations in Germany. While randomized controlled trials (RCTs) are the gold standard for demonstrating efficacy, RWE serves as a crucial complement by providing insights into long-term outcomes and real-world treatment effectiveness—factors that RCTs may not fully capture.
In line with these efforts, the German Federal Joint Committee (G-BA) has commissioned the Institute for Quality and Efficiency in Healthcare (IQWiG) to develop a methodology for real-world data collection in a rapid report. This initiative aims to advance the use of RWE by addressing key methodological challenges in nonrandomized comparative studies. IQWiG will evaluate best practices for identifying statistical confounders, estimating sample sizes, handling treatment switches and missing data, and applying propensity score analyses in small patient populations. Additionally, input from pharmaceutical companies and stakeholders is being encouraged to refine the methodological framework for RWE collection.
The United Kingdom: Embracing RWE for Innovation and Efficiency
The UK healthcare system, with its centralized NHS and the influential role of NICE, presents a unique context for understanding RWE’s role in market access. Although some terminology may sound drug-centric, the NICE framework for RWE explicitly applies to all health technologies, including MD&D. Stephen Duffield, PhD, MD, Associate Director for Real-World Evidence Methods at NICE, emphasized the increasing use of RWE in NICE appraisals, particularly for health technologies, including medical devices and digital health solutions, and in areas of unmet need. He highlighted the value of RWE in:
- RWE can support the comprehensive evaluation of long-term safety, performance, and use in diverse patient populations, which are critical parameters for MD&D.
- RWE can support late-stage assessment by monitoring real-world performance and identifying rare adverse events.
- NICE’s EVA program utilizes RWE to support the adoption of promising technologies while generating evidence.
Finally, the challenges associated with the United Kingdom’s diverse healthcare system, particularly in the devolved nations of England, Scotland, Wales, and Northern Ireland, were acknowledged. While there are national health policies and guidelines, healthcare delivery, budgeting, and adoption decisions occur at a regional level, which may vary across these nations and even regions. Additionally, the need for robust data standards and methodologies to ensure the reliability and validity of RWE was emphasized.
Real-world evidence can complement clinical trial evidence by defining unmet medical needs and refining risk assessment.
France: Navigating a Multifaceted System for MD&D
France, with its multifaceted healthcare system and a strong focus on clinical trials, presents a distinct landscape for utilizing RWE. Sandrine Bourguignon, CEO of RWEality, highlighted the importance of understanding the various pathways and committees involved in evaluating and reimbursing medical devices. While clinical trials remain the gold standard, RWE plays an important role in:
- RWE is frequently used to monitor the real-world performance and safety of medical devices. Additionally, real-world data is a requirement for innovative biological and in vitro devices under the new RIHN 2.0 framework, which was implemented as of November 2024.6
- RWE supports the identification of specific patient populations, their characteristics, and their needs.
- France is exploring the use of RWE to assess the organizational impacts of new technologies, considering cost savings and improvements in patient pathways.
Finally, the importance of proactive data collection, robust methodologies, and the availability of comprehensive data sources, such as the French National Health Insurance database, to support RWE studies was emphasized.
Overview of Country-Specific Key Information
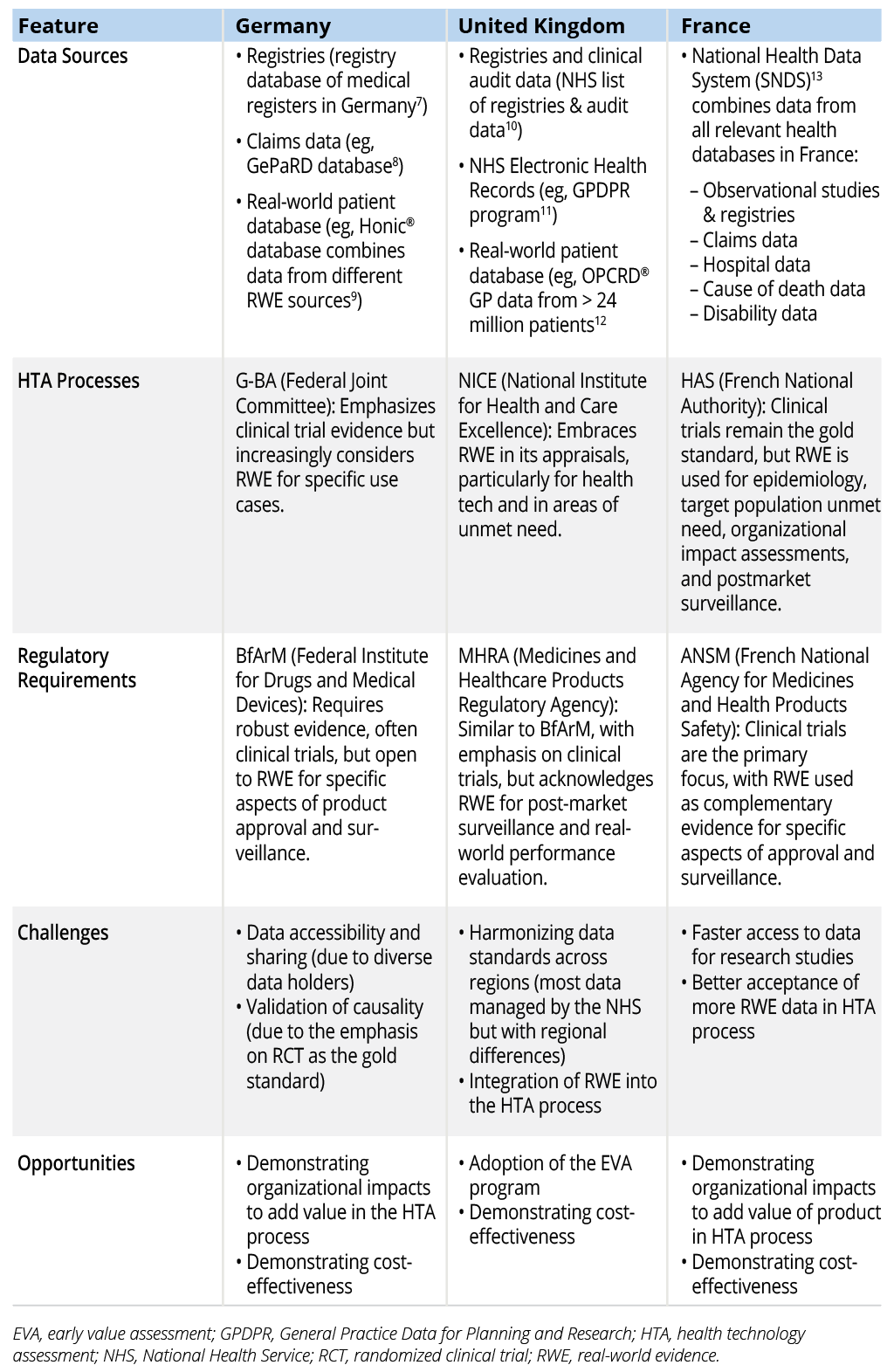
Table 1 provides a comparison of RWE approaches for market access in Germany, the United Kingdom, and France. This table provides a concise overview of the key differences and similarities in the approaches to RWE across the 3 countries, highlighting both convergence and divergence in policy and practice. It should help readers understand the nuanced landscape of RWE in Europe and identify key areas for improvement and harmonization.
Table 1. Comparison of RWE Approaches for Market Access in Germany, the United Kingdom, and France
Industry Perspective: Navigating a Complex Landscape
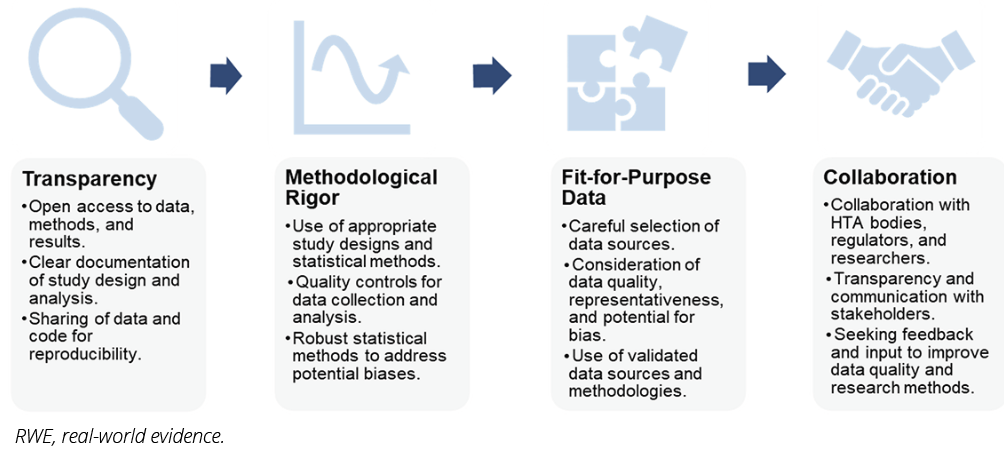
James Lavin, senior manager at Intuitive Surgical, provided a valuable industry perspective on utilizing RWE in a complex European market access landscape. He highlighted the importance of a structured approach to developing robust RWE, focusing on these key pillars:
- The research question should be specific, relevant to the target market, and aligned with the evidence needs of HTA bodies and other stakeholders. [Example: In France, RWE based on the SNDS database has been used to evaluate the long-term safety and performance of implantable cardiac devices such as pacemakers and defibrillators, supporting regulatory and reimbursement decisions.3]
- The choice of data sources should be carefully considered, ensuring that the data are of high quality, representative of the target population, and relevant to the research question. [Example: In Germany, a recent DTx for type 2 diabetes, which uses real-world data such as electronic health records and wearable device data, has been evaluated to support ongoing assessment and market access decisions.1]
- Statistical methods used to analyze RWE should be robust and statistically sound to address potential biases and ensure generalizability of findings. [Example: The United Kingdom’s EVA program employs rigorous methodologies, including the collection of observational and registry data, to support late-stage evaluations of health technologies, such as diagnostics and devices.5]
- Transparency throughout the research process is crucial, including clear documentation of methods, data sources, and analysis techniques. Sharing these details with HTA bodies and other stakeholders fosters trust in the research and its findings.
The importance of early engagement with HTA bodies, seeking their guidance on research questions, methods, and the types of evidence they are looking for, was emphasized. Collaboration with methodologists and other experts is also essential to validate research designs and ensure methodological rigor.
In practice, these principles are exemplified across Europe: in France through the use of SNDS data for implantable cardiac devices,3 in Germany through postmarket surveillance of wound dressings and diagnostics,1 and in the United Kingdom through the collection of RWE within the framework of the EVA program,5 which supports the evaluation of medical devices and diagnostics. These examples demonstrate how MD&D can be integrated into the RWE framework to support market access and life-cycle management.
These aspects are visualized in Figure 2. Each pillar is crucial for building trust in RWE and ensuring its value in supporting market access decisions.
Figure 2. Pillars of Robust RWE Development
Conclusion
The webinar highlighted the growing importance of RWE in navigating the complex landscape of MD&D market access in Europe. While each country faces unique challenges related to data availability, HTA processes, and regulatory requirements, there is a clear consensus that RWE can play a valuable role in bridging evidence gaps, informing decision making, and enhancing patient outcomes.
Proactive data collection, rigorous methodologies, and transparent communication are essential for building trust in real-world evidence.
Manufacturers have an important role to play in developing robust RWE strategies, ensuring that their research is aligned with the needs of HTA bodies and other stakeholders. Proactive data collection, rigorous methodologies, and transparent communication are essential for building trust in RWE and maximizing its value in supporting market access.
Collaboration is key. HTA bodies need to develop clear guidelines and processes for evaluating and incorporating RWE into their assessments. Policymakers must work collaboratively to establish data standards and facilitate data sharing, making RWE more readily accessible and reliable. By fostering a culture of collaboration and transparency, stakeholders can unlock the full potential of RWE to drive innovation, improve patient outcomes, and enhance the European market access landscape for medical devices and diagnostics.
Implications
- Manufacturers must proactively develop robust RWE strategies to support market access, including data collection, analysis, and communication.
- HTA bodies need to develop clear guidelines and processes for evaluating and incorporating RWE into their assessments.
- Policymakers should work collaboratively to establish data standards and facilitate data sharing, making RWE more readily accessible and reliable.
Lessons Learned
The webinar highlighted several key lessons learned for maximizing the value of RWE in MD&D market access:
- Data collection should be strategically planned early in the innovation process, ensuring the integration of real-world data elements into clinical trials, regulatory submissions, and postmarket surveillance to support continuous evidence generation throughout the product life-cycle.
- Carefully select real-world data sources relevant to the research question, considering data quality, representativeness, and potential for bias.
- Ensure that RWE studies are designed and conducted with methodological rigor, using appropriate statistical analysis to draw valid conclusions.
- Ensure transparency throughout the research process by systematically sharing methods and data with stakeholders, while fostering collaboration with HTA bodies, regulators, and academic researchers to enhance data quality, optimize processes, and support continuous evidence generation across the product life-cycle.
- Demonstrate the value of RWE to key stakeholders, emphasizing its ability to address evidence gaps, inform decision making, and ultimately improve patient outcomes.
References
- Kannenberg S, Voggel J, Thieme N, et al. Unlocking potential: personalized lifestyle therapy for type 2 diabetes through a predictive algorithm-driven digital therapeutic. J Diabetes Sci Technol. 2024:19322968241266821.
- National Institute for Health and Care Excellence. Transcatheter heart valves for transcatheter aortic valve implantation to treat aortic stenosis: late stage assessment. Published August 21, 2025. Accessed September 23, 2025. https://www.nice.org.uk/guidance/indevelopment/gid-hte10027
- de Pouvourville G, Armoiry X, Lavorel A, et al. Real-world data and evidence in health technology assessment: when are they complementary, substitutes, or the only sources of data compared to clinical trials? Therapie. 2023;78(1):81-94.
- Bundesministerium für Gesundheit. Sozialgesetzbuch (SGB) - Fünftes Buch (V) - Gesetzliche Krankenversicherung. Published December 20, 1988. Accessed March 3, 2025. https://www.gesetze-im-internet.de/sgb_5/
- National Institute for Health and Care Excellence. Early value assessment (EVA) for medtech. Accessed March 3, 2025. https://www.nice.org.uk/about/what-we-do/eva-for-medtech
- Haute Autorité de Santé (HAS). RIHN (Réseau d’Innovation en Santé Numérique) 2.0 Framework. Published December 6, 2024. Accessed on March 3, 2025. https://www.has-sante.fr/jcms/p_3566839/fr/deposer-une-demande-d-inscription-au-referentiel-des-actes-innovants-hors-nomenclature-rihn-2-0
- Institut für Qualität und Patientensicherheit (BQS) GmbH. Register database of medical registers in Germany. Accessed March 3, 2025. https://registersuche.bqs.de/search_ext.php
- Leibniz-Institut für Präventionsforschung und Epidemiologie (BIPS). Die pharmakoepidemiologische Forschungsdatenbank (GePaRD). Accessed March 3, 2025. https://www.bips-institut.de/forschung/forschungsinfrastrukturen/gepard.html
- Health Data Technologies. The German Real-World Data Platform - honic. Accessed March 3, 2025. https://honic.eu/de
- National Health Service (NHS) - England. List of national clinical databases, registries and audits. Published March 16, 2024. Accessed March 3, 2025. https://www.england.nhs.uk/publication/list-of-national-clinical-databases-registries-and-audits/
- National Health Service (NHS) - England. General Practice Data for Planning and Research (GPDPR). Accessed March 3, 2025. https://digital.nhs.uk/services/general-practice-data-for-planning-and-research
- Optimum Patient Care. Optimum Patient Care Research Database (opcrd). Updated January 23, 2025. Accessed March 3, 2025. https://opcrd.optimumpatientcare.org/
- Caisse Nationale de l’Assurance Maladie des Travailleurs Salariés (CNAMTS). Système National des Données de Santé (SNDS). Accessed March 3, 2025. https://www.snds.gouv.fr/SNDS/Qu-est-ce-que-le-SNDS

