Tools for Compensating Patients for Their Patient-Engagement Activities
Eleanor Perfetto, PhD, MS, National Health Council, Washington, DC, USA, University of Maryland School of Pharmacy; Silke Schoch, National Health Council, Washington, DC, USA; Elisabeth Oehrlein, PhD, MS, National Health Council, Washington, DC, USA
Introduction
Patient engagement in health-related research has become a mainstay over the past decade, recognized and promulgated by government, industry, and, of course, patients themselves. The formation of the Patient-Centered Outcomes Research Institute in 2010 has increased interest in patient-centered outcomes research.1 The value of the patient community’s perspective in successful medical product research is also well accepted with the US Food and Drug Administration and other regulators, promoting the engagement of patients and patient groups in all aspects of medical product development.2-5
One historical barrier to widespread patient engagement has been disagreement over whether or not to compensate patients for their contributions to patient engagement activities.6 More recently, there is generally broad agreement that, as experts in their condition, patients should be compensated for the expertise they contribute to drug development for companies. However, stakeholders still lack clarity regarding how, and how much, to compensate for which patient engagement activities.
Fair market value (FMV) calculators are tools that are traditionally used by industry to ensure the compensation rates they are using when engaging doctors, researchers, and other outside experts are both competitive and compliant with regulations. These methods for determining appropriate compensation for clinicians and researchers are not applicable for patients, as many were originally created for healthcare providers, using qualifications only applicable to them. Based on patient group and industry member feedback that an alternative process for determining FMV for patient engagement was needed, the National Health Council, in partnership with Patient-Focused Medicines Development, and guided by 2 advisory committees, developed the first toolbox on compensation for patient engagement activities to support compensation and reimbursement decisions.
The goal was to create a toolbox that would guide compensation of patients and patient groups who are involved in patient engagement activities, mostly related to medical product development.
How the Toolbox Was Developed
The goal was to create a toolbox that would guide compensation of patients and patient groups who are involved in patient engagement activities, mostly related to medical product development. The toolbox was not meant to be applied to patients involved in clinical trials or in advertising/marketing activities.
Steering and Review Committees
Two committees were formed to support the project work. The Fair Market Value Steering Committee assisted in providing strategic direction and guidance for the project. The steering committee comprised individuals who were knowledgeable about patient engagement and familiar with the compensation of patients, caregivers, and patient-advocacy groups. Throughout the content creation process, the steering committee reviewed the materials and helped to navigate any potential issues that arose in the project. The Fair Market Value Review Committee was dedicated solely to assessing and critically reviewing the content produced, which allowed for a deeper dive into the materials than the steering committee was asked to provide.
Partnerships
The National Healthcare Corporation (NHC) partnered with Patient Focused Medicines Development and the Workgroup of European Cancer Patient Advocacy Networks (WECAN) in tool development. The partners collaborated, shared information, and exchanged deliverables so that the processes and outputs would be efficient and aligned.
Interviews
The NHC team conducted 60 interviews to engage key stakeholders on the topic including, but not limited to, patient advocacy organizations, medical product companies (pharmaceutical, biopharmaceutical, diagnostic, and device), and other research organizations and nonprofits. The NHC also tapped the findings from surveys by the Patient Focused Medicines Development and the Workgroup of European Cancer Patient Advocacy Networks.7,8
Wage and Benefits Data
As there is no compensation benchmarking data for “patients,” the NHC used compensation benchmarks for positions requiring similar skills, such as hospital patient representatives, and research, marketing, and health education positions. The NHC also used its own 2019 annual compensation survey data to find the appropriate FMV rate for patient organization staff to estimate an hourly consulting rate. Benchmark annual compensation was adjusted to reflect independent consulting services and produce a rate that includes salary, benefits, overhead, and profit based on the market data. This annual compensation was then transformed into an hourly rate by dividing the number of work hours in a typical year.9,10
Definitions
It was important that all participants used the same definitions for terminology as we progressed through toolbox development. We used the US Physician Self-Referral (“Stark”) Regulation definition in our methodology for this project.9 We also created a glossary that defined for this project terms, including: individual patient, caregiver, family member, patient group representative, etc.11 All definitions used in the toolbox can be found here.
Patient Activities Framework
To outline all the patient engagement activities that patients could be involved in, the NHC developed a patient activities framework. To create the framework, the NHC adapted and consolidated an extensive activities list developed by Patient Focused Medicines Development. This document had more than 150 patient engagement activities identified from 20 unique sources.12 The NHC consolidated the activities into general categories such as cocreation, presentation, mock trial, interview, focus group, reviewer, advisory board member, recruit, etc.13 The steering and review committees refined it further and added or subtracted categories as appropriate.
Reviews and Public Comment Period
The NHC wanted to ensure its membership and the public were able to comment on foundational components of the project, the Compensation Principles and Contracting Principles. The NHC membership and the wider public received notice of the open comment period on the 2 documents in December 2019 with 1 month to submit comments. The 2 documents were refined based on the comments received.
Beta Testing of the Calculator
The NHC engaged 11 organizations to beta test the FMV calculator. These organizations included patient advocacy groups, medical product developers, membership organizations, and other research organizations. Once the beta testers returned their feedback via a questionnaire and survey tool, the calculator was further refined and finalized by the steering and review committees. See Table for the tools in the toolbox.
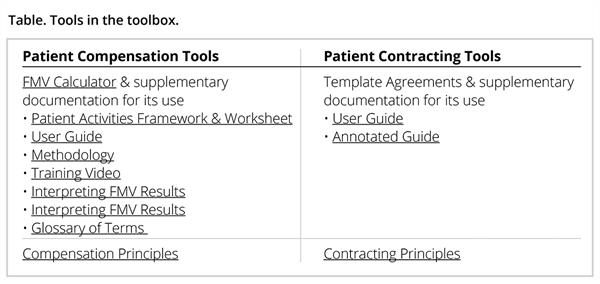
FMV Calculator
The purpose of the FMV Calculator is to provide a guide for patient advocates and medical product companies that enter into arrangements where the company is paying a patient or patient advocacy organization for professional services and expenses incurred in connection with the engagement.
The calculator is customizable and allows the user to choose14:
• Type of patient (eg, patient, caregiver, family member, patient advocate)
• Skills required for the engagement (eg, newly diagnosed with a specific disease)
• Specific activities the participant will be involved in (eg, input into a protocol)
• Expected time the participants will dedicate to the activity (eg, 1 8-hour day on 3 separate occasions)
• Modifiers that could appropriately alter the rate of compensation (eg, wages lost, the urgency of the work, or potential risks involved)
It should be noted the calculator is a guide only and is expected to be adapted by each user to their own needs. It is not intended to fix compensation rates. A range of rates is provided and the user selects a rate in that range based upon specific circumstances.
Compensation Principles
The Compensation Principles provide guidance on how and when to compensate patients, caregivers, and patient advocacy groups for their involvement in patient engagement activities.
The compensation principles cover the following areas15:
• Type of Patient Engagement Participant
• General Compensation
• Administrative/Logistics
• Time Commitment
• Travel and Reimbursement Considerations
• Declining Compensation
• Other Considerations
The principles provided the foundation for the compensation toolbox and guided considerations on such things as what to do when someone declines compensation, or when engaging a celebrity patient, how travel expenses should be reimbursed, and other similar issues.
The principles provided the foundation for the compensation toolbox and guided considerations on such things as what to do when someone declines compensation, or when engaging a celebrity patient, how travel expenses should be reimbursed, and other similar issues.
Contracting Principles and Template Agreements
In collaboration with the Patient Focused Medicines Development and the Workgroup of European Cancer Patient Advocacy Networks, the NHC adapted for use in the United States a set of European-focused legal agreements and contracting principles for interactions between stakeholders and the patient and caregiver community. The Contracting Principles provide guidance on creating agreements between patients and companies for patient engagement activities. The document uses examples to describe concepts like confidential information, intellectual property, data protection, indemnification, adverse event reporting, conflict of interest, and more.16
To create template agreements for meaningful partnerships between researchers and members of the patient advocacy community, the NHC adapted templates originally created for a project of the Workgroup of European Cancer Patient Advocacy Networks and the Myeloma Patients Europe that was produced with the Patient Focused Medicines Development and the “independent participation of over 10 pharmaceutical companies.”17 These 4 original template agreements were combined into 1 document and reviewed by a US-based legal team, who updated the documents in alignment with US law. After the template agreements were refined for a US audience, the steering and review committees reviewed them with their own legal and compliance teams and provided recommendations for further updates.
Conclusion and Next Steps
The NHC’s online FMV Calculator and other tools can be used to determine compensation for patients, caregivers, and patient groups involved in patient engagement activities taking place between patient organizations and/or individual patients and private companies. Use of the calculator can ensure that patients are fairly compensated and promote consistency across companies. While these tools are currently only available for compensation in the United States, the NHC’s partner on this project, the Patient Focused Medicines Development, will soon be adapting the tools for use in Europe.
As a final note, these tools also acknowledge that patients (and caregivers and patient advocates) are experts on the diseases they live with every day and deserve to be compensated as such.
Acknowledgment: The calculator was created by the NHC with support from Allergan, Biogen, Boehringer Ingelheim, Bristol Myers Squibb (sponsorship under Celgene), Grifols, Johnson & Johnson, Merck, Novartis, Pfizer, PFMD, Sangamo, Servier, and UCB.
We acknowledge the contributions of our steering and review committees:
Steering Committee:
Kate Avery, MPH Beyond Celiac
John Boyle, MA, & Lynn Albizo Immune Deficiency Foundation
Nicholas Brooke Patient Focused Medicines Development
Barbara Collura RESOLVE: The National Infertility Association
Louisa M. Daniels, JD, MBA Pfizer
Tracy Hart Osteogenesis Imperfecta Foundation
Ellen Ivey & Katherine Capperella Janssen Pharmaceuticals, Johnson & Johnson
Jan Nissen Merck
Amber Spierer, MPH Novartis
Louise Vetter Huntington’s Disease Society of America
Review Committee:
Rebekah Angove, PhD Patient Advocate Foundation
Julie Eller Arthritis Foundation
Jason Harris Lupus Foundation of America
Chris Healey Grifols
Dory Kranz National Alopecia Areata Foundation
Nancy Law Myasthenia Gravis Foundation of America
Kristi Lengyel, MBA, & Courtney George UCB, Inc
Christeen Moburg Sangamo
Karen M. Morales University of Maryland School of Pharmacy
Susan Stone, MBA The Allergan Foundation; Trustee, Allergan International Foundation
Keri Yale, MBA Boehringer Ingelheim Pharmaceuticals
References
1. Forsythe L, Heckert A, Margolis MK, Schrandt S, Frank L. Methods and impact of engagement in research, from theory to practice and back again: early findings from the Patient-Centered Outcomes Research Institute. Qual Life Res. 2018;27(1):17-31. doi:10.1007/s11136-017-1581-x.
2. Hoos A, Anderson J, Boutin M, et al. Partnering with patients in the development and lifecycle of medicines: a call for action. Ther Innov Regul Sci. 2015;49(6):929-939. doi:10.1177/2168479015580384.
3. FDA and European Medicines Agency Patient Engagement Cluster. FDA. Published April 18, 2019. https://www.fda.gov/patients/learn-about-fda-patient-engagement/fda-and-european-medicines-agency-patient-engagement-cluster. Accessed July 22, 2019.
4. Chalasani M, Vaidya P, Mullin T. Enhancing the incorporation of the patient’s voice in drug development and evaluation. Res Involv Engagem. 2018;4(1):10. doi:10.1186/s40900-018-0093-3.
5. ICH Reflection Paper. Proposed ICH Guideline Work to Advance Patient Focused Drug Development. Published 2021. https://admin.ich.org/sites/default/files/2020-12/ICH_ReflectionPaper_PFDD_Endorsed-ForConsultation_2020_1118.pdf. Accessed May 2, 2021.
6. Richards DP, Jordan I, Strain K, Press Z. Patient partner compensation in research and health care: the patient perspective on why and how. Patient Experience J. 2018;5(3). doi:10.35680/2372-0247.1334.
7. Patient Focused Medicines Development. Compensation for Patient Experts at Fair Market Value: Tackling Together the Patient Engagement Hot Topic. https://patientfocusedmedicine.org/fair-market-value/ Accessed April 15, 2021.
8. Geissler J, Plate A, Spurrier G, Taylor J, Oliver K, Oliver G. Compensation for patient experts at fair market value. Presented October 28, 2018. https://wecanadvocate.eu/wp-content/uploads/2019/02/2018-10-28-WECAN-Fair-Market-Value-Project-and-Survey-Result-1.2.pdf Accessed April 15, 2021.
9. National Health Council. Fair Market Value Hourly Rate Methodology. Published online 2020. https://nationalhealthcouncil.org/wp-content/uploads/2020/06/FMV_Hourly_Rate_Methodology.pdf Accessed June 26, 2020.
10. Perfetto E, Schoch S. NHC Fair Market Value Calculator Demonstration Webinar. Published 2020. https://nationalhealthcouncil.org/nhc-fair-market-value-calculator-demonstration-webinar/ Accessed September 30, 2021.
11. National Health Council. NHC Fair Market Value Glossary of Terms. Published online 2020. https://nationalhealthcouncil.org/wp-content/uploads/2020/06/FMV_Glossary_Terms.pdf Accessed June 26, 2020.
12. Patient Engagement Synapse. 150+ PE Activities to Inform PE Quality Guidance’s How to Modules. Updated June 18, 2020. https://synapse.pfmd.org/initiatives/150-pe-activities-to-inform-pe-quality-guidances-how-to-modules. Accessed May 2, 2021.
13. National Health Council. Patient Activities Framework. Published 2020. https://nationalhealthcouncil.org/wp-content/uploads/2020/06/NHC_FMV_Activities_List.pdf Accessed June 26, 2020.
14. National Health Council. National Health Council Patient Engagement Fair Market Value Calculator. Published 2020. https://nationalhealthcouncil.org/fair-market-value-calculator/ Accessed June 26, 2020.
15. National Health Council. The National Health Council Principles for Compensating Patients for Patient Engagement Activities. Published 2020. https://nationalhealthcouncil.org/wp-content/uploads/2021/03/NHC_FMV_Patient_Engagement_Compensation-Principles.pdf Accessed June 26, 2020.
16. National Health Council. Principles on Contracting Between Patient Advocates and Pharmaceutical Companies. Published 2020. https://nationalhealthcouncil.org/wp-content/uploads/2021/03/Contracting-Principles-Document-_03222021-ss-ts.pdf Accessed April 8, 2021.
17. Reasonable Legal Agreements – 2020 —Patient Focused Medicines Development. https://patientfocusedmedicine.org/reasonable-legal-agreements/. Accessed July 24, 2019.

